Protein kinase C-theta mediates negative feedback on regulatory T cell function
- PMID: 20339032
- PMCID: PMC2905626
- DOI: 10.1126/science.1186068
Protein kinase C-theta mediates negative feedback on regulatory T cell function
Erratum in
- Science. 2010 May 21;328(5981):974. doi: 10.1126/science.328.5981.974-b doi: 10.1126/science.328.5981.974-b
Abstract
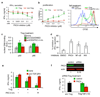
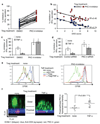
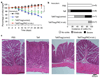
T cell receptor (TCR)-dependent regulatory T cell (Treg) activity controls effector T cell (Teff) function and is inhibited by the inflammatory cytokine tumor necrosis factor-alpha (TNF-alpha). Protein kinase C-theta (PKC-theta) recruitment to the immunological synapse is required for full Teff activation. In contrast, PKC-theta was sequestered away from the Treg immunological synapse. Furthermore, PKC-theta blockade enhanced Treg function, demonstrating PKC-theta inhibits Treg-mediated suppression. Inhibition of PKC-theta protected Treg from inactivation by TNF-alpha, restored activity of defective Treg from rheumatoid arthritis patients, and enhanced protection of mice from inflammatory colitis. Treg freed of PKC-theta-mediated inhibition can function in the presence of inflammatory cytokines and thus have therapeutic potential in control of inflammatory diseases.
Figures

Comment in
-
Regulatory T-cell function in autoimmunity.Immunotherapy. 2010 Jul;2(4):441-2. doi: 10.2217/imt.10.37. Immunotherapy. 2010. PMID: 20635997 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

