Validation of a predictive model for identifying an increased risk for thromboembolism in children with acute lymphoblastic leukemia: results of a multicenter cohort study
- PMID: 20339086
- PMCID: PMC2890143
- DOI: 10.1182/blood-2010-01-263012
Validation of a predictive model for identifying an increased risk for thromboembolism in children with acute lymphoblastic leukemia: results of a multicenter cohort study
Abstract
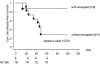
Among risk factors for developing thromboembolism (VTE) in children with acute lymphoblastic leukemia were Escherichia coli asparaginase, concomitant steroid use, presence of central venous lines, and thrombophilic abnormalities. Developing a predictive model for determining children at increased risk would be beneficial in targeting interventional studies to high-risk groups (HRGs). Predictive variables were incorporated into a risk assessment model, which was evaluated in 456 children and then validated in 339 patients. VTE risk by score was no greater than 2.5 for low-risk group (LRG) and greater than 2.5 for HRG. VTE rates at 3.5 months (validation cohorts) were 2.5% in LRG and 64.7% in HRG. In multivariate analysis adjusted for age, duration of asparaginase administration, enoxaparin prophylaxis, and T-immunophenotype, the HRG was significantly associated with VTE compared with the LRG (hazard/95% confidence interval [CI], 8.22/1.85-36.53). Model specificity was 96.2% and sensitivity was 63.2%. As secondary objective we investigated the use of enoxaparin for VTE prophylaxis in the HRG. HRG patients without enoxaparin prophylaxis showed a significantly reduced thrombosis-free survival compared with children on low-molecular-weight heparin (LMWH). On the basis of the high specificity, the model may identify children with leukemia at risk of VTE. LMWH may help prevent VTE in the HRG; this warrants assessment in larger cooperative clinical trials.
Figures



References
-
- Athale U, Siciliano S, Thabane L, et al. Epidemiology and clinical risk factors predisposing to thromboembolism in children with cancer. Pediatr Blood Cancer. 2008;51(6):792–797. - PubMed
-
- Caruso V, Iacoviello L, Di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–2222. - PubMed
-
- Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol. 2007;138(4):430–445. - PubMed
-
- Mitchell LG, Andrew M, Hanna K, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer. 2003;97(2):508–516. - PubMed
-
- Male C, Chait P, Ginsberg JS, et al. Comparison of venography and ultrasound for the diagnosis of asymptomatic deep vein thrombosis in the upper body in children: results of the PARKAA study. Prophylactic Antithrombin Replacement in Kids with ALL treated with Asparaginase. Thromb Haemost. 2002;87(4):593–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

