Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells
- PMID: 20339095
- PMCID: PMC2890142
- DOI: 10.1182/blood-2009-03-213363
Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells
Abstract
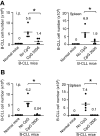
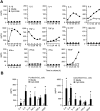
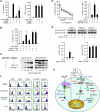
Chronic lymphocytic leukemia (CLL) is the most prevalent human leukemia and is characterized by the progressive accumulation of long-lived malignant B cells. Here we show that human B-CLL cells selectively express high levels of Toll-like receptor 9 (TLR9) mRNA and proteins. Treating B-CLL cells with TLR9 agonists, type B CpG oligodeoxynucleotides (CpG-B ODNs), induces significant morphologic and phenotypic activation, altered cytokine production, reversal of signal transducer, and activator of transcription 1 (STAT1) phosphorylation state, followed by profound apoptosis of B-CLL cells that is CpG-B ODN treatment time- and dose-dependent. TLR9-CpG ODN ligation-induced apoptosis of B-CLL cells is confirmed by viable cell counts, annexin V/propidium iodide and tetramethyl-rhodamine ethylester staining, Western blots of the activation, and cleaved caspases and poly (ADP-ribose) polymerase. Triggering TLR9 by CpG-B ODN leads to nuclear factor-kappaB-dependent production of autocrine interleukin-10, which activates JAK/STAT pathway-dependent tyrosine phosphorylation of STAT1 proteins and thereby provokes an apoptosis pathway in B-CLL cells. Treating B-CLL cells in vitro or in vivo with CpG-B ODN reduces the number of leukemia cells that engraft in NOD-scid mice. These findings provide new understanding of CpG ODN-mediated antitumor effects and support for the development of TLR9-targeted therapy for human CLL.
Figures


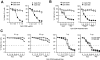

Similar articles
-
Immunostimulatory oligodeoxynucleotides induce apoptosis of B cell chronic lymphocytic leukemia cells.J Leukoc Biol. 2005 Mar;77(3):378-87. doi: 10.1189/jlb.0604373. Epub 2004 Dec 6. J Leukoc Biol. 2005. PMID: 15582984
-
Toll-like receptor agonists induce apoptosis in mouse B-cell lymphoma cells by altering NF-κB activation.Cell Mol Immunol. 2013 Jul;10(4):360-72. doi: 10.1038/cmi.2013.14. Epub 2013 Jun 3. Cell Mol Immunol. 2013. PMID: 23727784 Free PMC article.
-
CpG oligodeoxynucleotide CpG-685 upregulates functional interleukin-21 receptor on chronic lymphocytic leukemia B cells through an NF-κB mediated pathway.Oncotarget. 2015 Jun 30;6(18):15931-9. doi: 10.18632/oncotarget.3285. Oncotarget. 2015. PMID: 26158860 Free PMC article.
-
Immunostimulatory Activities of CpG-Oligodeoxynucleotides in Teleosts: Toll-Like Receptors 9 and 21.Front Immunol. 2019 Feb 8;10:179. doi: 10.3389/fimmu.2019.00179. eCollection 2019. Front Immunol. 2019. PMID: 30800129 Free PMC article. Review.
-
Effect of immunostimulatory CpG-oligonucleotides in chronic lymphocytic leukemia B cells.Leuk Lymphoma. 2001 Jul;42(3):301-7. doi: 10.3109/10428190109064586. Leuk Lymphoma. 2001. PMID: 11699394 Review.
Cited by
-
Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases.Int J Mol Sci. 2021 Dec 13;22(24):13397. doi: 10.3390/ijms222413397. Int J Mol Sci. 2021. PMID: 34948194 Free PMC article. Review.
-
Extracellular vesicles released from chronic lymphocytic leukemia cells exhibit a disease relevant mRNA signature and transfer mRNA to bystander cells.Haematologica. 2017 Mar;102(3):e100-e103. doi: 10.3324/haematol.2016.153197. Epub 2016 Nov 17. Haematologica. 2017. PMID: 27856511 Free PMC article. No abstract available.
-
Toll-like Receptors in Chronic Lymphocytic Leukemia.Mediterr J Hematol Infect Dis. 2012;4(1):e2012055. doi: 10.4084/MJHID.2012.055. Epub 2012 Aug 9. Mediterr J Hematol Infect Dis. 2012. PMID: 22973499 Free PMC article.
-
Lymphoma B-cell responsiveness to CpG-DNA depends on the tumor microenvironment.J Exp Clin Cancer Res. 2013 Apr 5;32(1):18. doi: 10.1186/1756-9966-32-18. J Exp Clin Cancer Res. 2013. PMID: 23561041 Free PMC article.
-
Protective Effects of Vitamin K Compounds on the Proteomic Profile of Osteoblasts under Oxidative Stress Conditions.Molecules. 2020 Apr 23;25(8):1990. doi: 10.3390/molecules25081990. Molecules. 2020. PMID: 32340397 Free PMC article.
References
-
- O'Brien S, del Giglio A, Keating M. Advances in the biology and treatment of B-cell chronic lymphocytic leukemia. Blood. 1995;85(2):307–318. - PubMed
-
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. - PubMed
-
- Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351(9):893–901. - PubMed
-
- Montserrat E, Moreno C, Esteve J, Urbano-Ispizua A, Gine E, Bosch F. How I treat refractory CLL. Blood. 2006;107(4):1276–1283. - PubMed
-
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

