C-reactive protein-mediated vascular injury requires complement
- PMID: 20339115
- PMCID: PMC2897052
- DOI: 10.1161/ATVBAHA.110.205377
C-reactive protein-mediated vascular injury requires complement
Abstract
Background: We previously demonstrated that vascular injury-induced neointima formation is exaggerated in human C-reactive protein (CRP) transgenic (CRPtg) compared to nontransgenic (NTG) mice. We now test the hypothesis that complement is required for this effect.
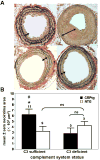
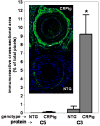
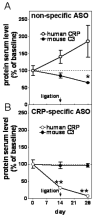
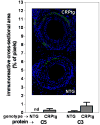
Methods and results: CRPtg and NTG with a normal complement system versus their counterparts lacking expression of complement component 3 (C3) protein (CRPtg/C3(-/-) and NTG/C3(-/-)) underwent carotid artery ligation. Twenty-eight days later, the injured vessels in CRPtg had thicker neointimas and more immunoreactive C3 in the surrounding adventitia compared with NTG. In CRPtg/C3(-/-), there was no increase in neointimal thickness compared with NTG or NTG/C3(-/-). Decreasing human CRP blood levels through administration of a selective antisense oligonucleotide eliminated the depletion of serum C3 associated with vascular injury and reduced immunoreactive C3 in the resultant lesions. In injured vessels, C3 colocalized with F4/80 (macrophage marker), and in vitro, human CRP elicited increased expression of C3 by bone marrow-derived macrophages.
Conclusions: Human CRP exaggeration of neointima formation in injured mouse carotid arteries associates with decreased circulating C3 and increased tissue-localized C3. C3 elimination or pharmacological reduction of human CRP prevents CRP-driven exacerbation of the injury response. In the CRPtg model system, mouse C3 is essential for the effect of human CRP.
Figures


References
-
- Wang D, Oparil S, Chen YF, McCrory MA, Skibinski GA, Feng W, Szalai AJ. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2094–2099. - PubMed
-
- Stein MP, Mold C, Du Clos TW. C-reactive protein binding to murine leukocytes requires Fc gamma receptors. J Immunol. 2000;164:1514–1520. - PubMed
-
- Hage FG, McCrory MA, Szalai AJ. C-reactive protein and cardiovascular disease: Lessons learned from studying genetically engineered mice. In: Nagasawa S, editor. C-Reactive Protein – New Research. Hauppauge NY: Nova Publishers; 2008. pp. 83–116.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

