3D-Reconstructions and Virtual 4D-Visualization to Study Metamorphic Brain Development in the Sphinx Moth Manduca Sexta
- PMID: 20339481
- PMCID: PMC2845058
- DOI: 10.3389/fnsys.2010.00007
3D-Reconstructions and Virtual 4D-Visualization to Study Metamorphic Brain Development in the Sphinx Moth Manduca Sexta
Abstract
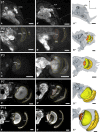
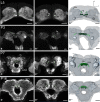
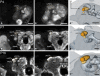
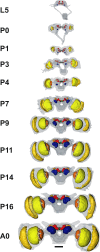
DURING METAMORPHOSIS, THE TRANSITION FROM THE LARVA TO THE ADULT, THE INSECT BRAIN UNDERGOES CONSIDERABLE REMODELING: new neurons are integrated while larval neurons are remodeled or eliminated. One well acknowledged model to study metamorphic brain development is the sphinx moth Manduca sexta. To further understand mechanisms involved in the metamorphic transition of the brain we generated a 3D standard brain based on selected brain areas of adult females and 3D reconstructed the same areas during defined stages of pupal development. Selected brain areas include for example mushroom bodies, central complex, antennal- and optic lobes. With this approach we eventually want to quantify developmental changes in neuropilar architecture, but also quantify changes in the neuronal complement and monitor the development of selected neuronal populations. Furthermore, we used a modeling software (Cinema 4D) to create a virtual 4D brain, morphing through its developmental stages. Thus the didactical advantages of 3D visualization are expanded to better comprehend complex processes of neuropil formation and remodeling during development. To obtain datasets of the M. sexta brain areas, we stained whole brains with an antiserum against the synaptic vesicle protein synapsin. Such labeled brains were then scanned with a confocal laser scanning microscope and selected neuropils were reconstructed with the 3D software AMIRA 4.1.
Keywords: Manduca; animation; brain; development; digital neuroanatomy; insect; neuropil.
Figures




Similar articles
-
Development and steroid regulation of RFamide immunoreactivity in antennal-lobe neurons of the sphinx moth Manduca sexta.J Exp Biol. 2004 Jun;207(Pt 14):2389-400. doi: 10.1242/jeb.01036. J Exp Biol. 2004. PMID: 15184511
-
Anisometric brain dimorphism revisited: Implementation of a volumetric 3D standard brain in Manduca sexta.J Comp Neurol. 2009 Nov 10;517(2):210-25. doi: 10.1002/cne.22150. J Comp Neurol. 2009. PMID: 19731336
-
Synaptic organization of the uniglomerular projection neurons of the antennal lobe of the moth Manduca sexta: a laser scanning confocal and electron microscopic study.J Comp Neurol. 1997 Mar 3;379(1):2-20. doi: 10.1002/(sici)1096-9861(19970303)379:1<2::aid-cne2>3.0.co;2-8. J Comp Neurol. 1997. PMID: 9057110
-
A novel serotonin-immunoreactive neuron in the antennal lobe of the sphinx moth Manduca sexta persists throughout postembryonic life.J Neurobiol. 1987 Sep;18(5):451-65. doi: 10.1002/neu.480180506. J Neurobiol. 1987. PMID: 3309187 Review.
-
Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster.Insect Biochem Mol Biol. 2003 Dec;33(12):1327-38. doi: 10.1016/j.ibmb.2003.06.001. Insect Biochem Mol Biol. 2003. PMID: 14599504 Review.
Cited by
-
Molecular interaction of fenvalarate with actin.Bioinformation. 2011;7(5):234-8. doi: 10.6026/97320630007234. Epub 2011 Oct 31. Bioinformation. 2011. PMID: 22125391 Free PMC article.
-
Age estimation during the blow fly intra-puparial period: a qualitative and quantitative approach using micro-computed tomography.Int J Legal Med. 2017 Sep;131(5):1429-1448. doi: 10.1007/s00414-017-1598-2. Epub 2017 May 4. Int J Legal Med. 2017. PMID: 28474172 Free PMC article.
-
Visualization of insect metamorphosis.Philos Trans R Soc Lond B Biol Sci. 2019 Oct 14;374(1783):20190071. doi: 10.1098/rstb.2019.0071. Epub 2019 Aug 26. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31438819 Free PMC article. Review.
-
The Role of Cells in Encoding and Storing Information: A Narrative Review of Cellular Memory.Cureus. 2024 Nov 5;16(11):e73063. doi: 10.7759/cureus.73063. eCollection 2024 Nov. Cureus. 2024. PMID: 39640131 Free PMC article. Review.
-
Controlling feeding behavior by chemical or gene-directed targeting in the brain: what's so spatial about our methods?Front Neurosci. 2013 Dec 18;7:182. doi: 10.3389/fnins.2013.00182. Front Neurosci. 2013. PMID: 24385950 Free PMC article. Review.
References
-
- Amos T. M., Gelman D. B., Mesce K. A. (1996). Steroid hormone fluctuations regulate ganglionic fusion during metamorphosis of the moth Manduca sexta. J. Insect Physiol. 42, 579–59110.1016/0022-1910(95)00127-1 - DOI
-
- Amos T. M., Mesce K. A. (1994). Reorganization of the ventral nerve cord in the moth Manduca sexta (L.) (Lepidoptera:Sphingidae). Int. J. Insect Morphol. Embryol. 23, 21–3710.1016/0020-7322(94)90013-2 - DOI
-
- Bell R. A., Joachim F. A. (1978). Techniques for rearing laboratory colonies of the tobacco hornworm, Manduca sexta, and pink ballworms. Ann. Entomol. Soc. Am. 69, 365–373
-
- Bullock T. H., Horridge G. A. (1965). Structure and Function in the Nervous Systems of Invertebrates, Vol. 2 San Francisco, Freeman
LinkOut - more resources
Full Text Sources

