3D Standard Brain of the Red Flour Beetle Tribolium Castaneum: A Tool to Study Metamorphic Development and Adult Plasticity
- PMID: 20339482
- PMCID: PMC2845059
- DOI: 10.3389/neuro.06.003.2010
3D Standard Brain of the Red Flour Beetle Tribolium Castaneum: A Tool to Study Metamorphic Development and Adult Plasticity
Abstract
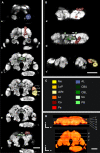
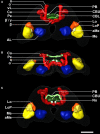
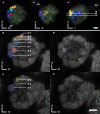
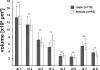
The red flour beetle Tribolium castaneum is emerging as a further standard insect model beside Drosophila. Its genome is fully sequenced and it is susceptible for genetic manipulations including RNA-interference. We use this beetle to study adult brain development and plasticity primarily with respect to the olfactory system. In the current study, we provide 3D standard brain atlases of freshly eclosed adult female and male beetles (A0). The atlases include eight paired and three unpaired neuropils including antennal lobes (ALs), optic lobe neuropils, mushroom body calyces and pedunculi, and central complex. For each of the two standard brains, we averaged brain areas of 20 individual brains. Additionally, we characterized eight selected olfactory glomeruli from 10 A0 female and male beetles respectively, which we could unequivocally recognize from individual to individual owing to their size and typical position in the ALs. In summary, comparison of the averaged neuropil volumes revealed no sexual dimorphism in any of the reconstructed neuropils in A0 Tribolium brains. Both, the female and male 3D standard brain are also used for interspecies comparisons, and, importantly, will serve as future volumetric references after genetical manipulation especially regarding metamorphic development and adult plasticity.
Keywords: antennal lobe; brain; coleoptera; digital neuroanatomy; insect; neuropil; olfactory system.
Figures


References
-
- Anton S., Homberg U. (1999). Antennal lobe structure. In Insect Olfaction, Hansson B. S., ed. (Berlin, Springer; ), pp. 97–124
-
- Arnold G., Masson C., Budharugsa S. (1984). Demonstration of a sexual dimorphism in the olfactory pathways of the drones of Apis mellifica L. (Hymenoptera, Apidae). Experientia 40, 723–725 10.1007/BF01949744 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

