Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer
- PMID: 20339913
- PMCID: PMC2855860
- DOI: 10.1007/s10549-010-0788-0
Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer
Abstract
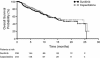
This multicenter, randomized, open-label phase III trial (planned enrollment: 700 patients) was conducted to test the hypothesis that single-agent sunitinib improves progression-free survival (PFS) compared with capecitabine as treatment for advanced breast cancer (ABC). Patients with HER2-negative ABC that recurred after anthracycline and taxane therapy were randomized (1:1) to sunitinib 37.5 mg/day or capecitabine 1,250 mg/m(2) (1,000 mg/m(2) in patients >65 years) BID on days 1-14 q3w. The independent data-monitoring committee (DMC) determined during the first interim analysis (238 patients randomized to sunitinib, 244 to capecitabine) that the trial be terminated due to futility in reaching the primary endpoint. No statistical evidence supported the hypothesis that sunitinib improved PFS compared with capecitabine (one-sided P = 0.999). The data indicated that PFS was shorter with sunitinib than capecitabine (median 2.8 vs. 4.2 months, respectively; HR, 1.47; 95% CI, 1.16-1.87; two-sided P = 0.002). Median overall survival (15.3 vs. 24.6 months; HR, 1.17; two-sided P = 0.350) and objective response rates (11 vs. 16%; odds ratio, 0.65; P = 0.109) were numerically inferior with sunitinib versus capecitabine. While no new or unexpected safety findings were reported, sunitinib treatment was associated with higher frequencies and greater severities of many common adverse events (AEs) compared with capecitabine, resulting in more temporary discontinuations due to AEs with sunitinib (66 vs. 51%). The relative dose intensity was lower with sunitinib than capecitabine (73 vs. 95%). Based on these efficacy and safety results, sunitinib should not be used as monotherapy for patients with ABC.
Figures
References
-
- Blackwell KL, Burstein HJ, Sledge GW et al (2009) Updated survival analysis of a randomized study of lapatinib alone or in combination with trastuzumab in women with HER2-positive metastatic breast cancer progressing on trastuzumab therapy. In: San Antonio Breast Cancer symposium (Abstract 61)
-
- Roche Pharmaceuticals (2006) XELODA (capecitabine) prescribing information, Nutley, NJ
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous