The role of population PK-PD modelling in paediatric clinical research
- PMID: 20340012
- PMCID: PMC3082690
- DOI: 10.1007/s00228-009-0782-9
The role of population PK-PD modelling in paediatric clinical research
Abstract
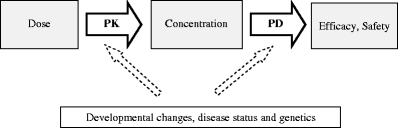
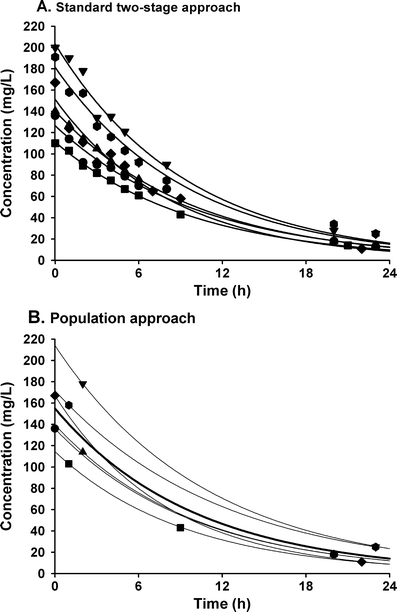
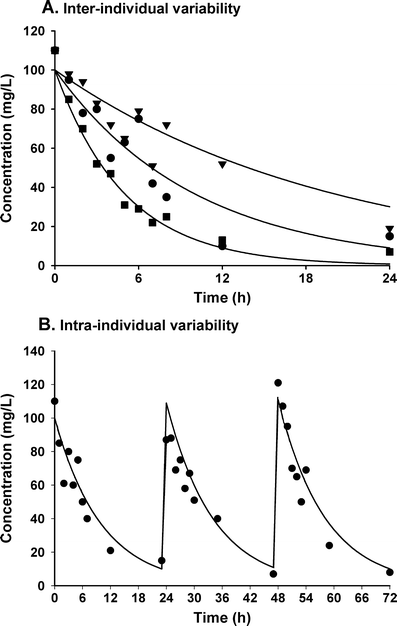
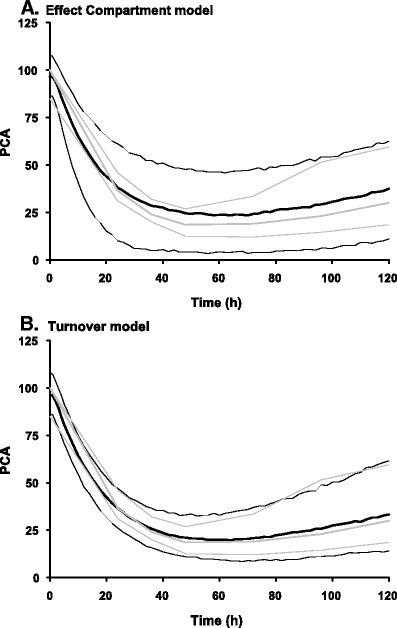
Children differ from adults in their response to drugs. While this may be the result of changes in dose exposure (pharmacokinetics [PK]) and/or exposure response (pharmacodynamics [PD]) relationships, the magnitude of these changes may not be solely reflected by differences in body weight. As a consequence, dosing recommendations empirically derived from adults dosing regimens using linear extrapolations based on body weight, can result in therapeutic failure, occurrence of adverse effect or even fatalities. In order to define rational, patient-tailored dosing schemes, population PK-PD studies in children are needed. For the analysis of the data, population modelling using non-linear mixed effect modelling is the preferred tool since this approach allows for the analysis of sparse and unbalanced datasets. Additionally, it permits the exploration of the influence of different covariates such as body weight and age to explain the variability in drug response. Finally, using this approach, these PK-PD studies can be designed in the most efficient manner in order to obtain the maximum information on the PK-PD parameters with the highest precision. Once a population PK-PD model is developed, internal and external validations should be performed. If the model performs well in these validation procedures, model simulations can be used to define a dosing regimen, which in turn needs to be tested and challenged in a prospective clinical trial. This methodology will improve the efficacy/safety balance of dosing guidelines, which will be of benefit to the individual child.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

