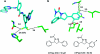
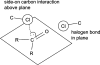
A medicinal chemist's guide to molecular interactions
- PMID: 20345171
- PMCID: PMC2905122
- DOI: 10.1021/jm100112j
A medicinal chemist's guide to molecular interactions
Erratum in
- J Med Chem. 2010 Aug 26;53(16):6241
Figures
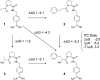

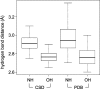
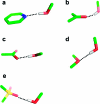



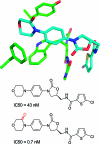
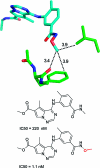

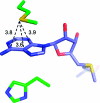
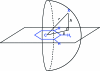
References
-
- Allen F. H. The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr. 2002, B58, 380–388. - PubMed
-
- Bernstein F. C.; Koetzle T. E.; Williams G. J. B.; Meyer J., E. F.; Brice M. D.; Rodgers J. R.; Kennard O.; Shimanouchi T.; Tasumi M. The protein data bank: a computer-based archival file for macromolecular structures. J. Mol. Biol. 1977, 112, 535–542; http://www.rcsb.org/pdb/. - PubMed
-
- Mark A. E.; van Gunsteren W. F. Decomposition of the free energy of a system in terms of specific interactions. Implications for theoretical and experimental studies. J. Mol. Biol. 1994, 240, 167–176. - PubMed
-
- Dill K. A. Additivity principles in biochemistry. J. Biol. Chem. 1997, 272, 701–704. - PubMed
-
- Olsson T. S. G.; Williams M. A.; Pitt W. R.; Ladbury J. E. The thermodynamics of protein−ligand interaction and solvation: insights for ligand design. J. Mol. Biol. 2008, 384, 1002–1017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

