Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery
- PMID: 20345327
- PMCID: PMC2858262
- DOI: 10.1517/17425240903571614
Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery
Abstract
Importance of the field: Plasmonic nanoparticles provide a new route to treat cancer owing to their ability to convert light into heat effectively for photothermal destruction. Combined with the targeting mechanisms possible with nanoscale materials, this technique has the potential to enable highly targeted therapies to minimize undesirable side effects.
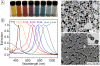
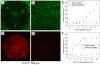
Areas covered in this review: This review discusses the use of gold nanocages, a new class of plasmonic nanoparticles, for photothermal applications. Gold nanocages are hollow, porous structures with compact sizes and precisely controlled plasmonic properties and surface chemistry. Also, a recent study of gold nanocages as drug-release carriers by externally controlling the opening and closing of the pores with a smart polymer whose conformation changes at a specific temperature is discussed. Release of the contents can be initiated remotely through near-infrared irradiation. Together, these topics cover the years from 2002 to 2009.
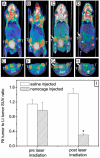
What the reader will gain: The reader will be exposed to different aspects of gold nanocages, including synthesis, surface modification, in vitro studies, initial in vivo data and perspectives on future studies.
Take home message: Gold nanocages are a promising platform for cancer therapy in terms of both photothermal destruction and drug delivery.
Figures


References
-
-
Riehemann K, Schneider SW, Luger TA, et al. Nanomedicine - challenge and perspectives. Angew Chem Int Ed. 2009;48:872–897. ** Broad overview of recent advances and future perspectives in the field of nanomedicine.
-
-
-
Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotech. 2007;2:751–60. ** Broad overview of ways nanotechnology can be used for cancer treatment, including the current development stage of different therapies.
-
-
- Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. - PubMed
-
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
