The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity
- PMID: 20345659
- PMCID: PMC2906755
- DOI: 10.1111/j.1365-2958.2010.07135.x
The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity
Abstract
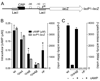
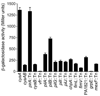
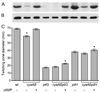
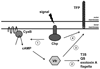
Multiple virulence systems in the opportunistic pathogen Pseudomonas aeruginosa are regulated by the second messenger signalling molecule adenosine 3', 5'-cyclic monophosphate (cAMP). Production of cAMP by the putative adenylate cyclase enzyme CyaB represents a critical control point for virulence gene regulation. To identify regulators of CyaB, we screened a transposon insertion library for mutants with reduced intracellular cAMP. The majority of insertions resulting in reduced cAMP mapped to the Chp gene cluster encoding a putative chemotaxis-like chemosensory system. Further genetic analysis of the Chp system revealed that it has both positive and negative effects on intracellular cAMP and that it regulates cAMP levels by modulating CyaB activity. The Chp system was previously implicated in the production and function of type IV pili (TFP). Given that cAMP and the cAMP-dependent transcriptional regulator Vfr control TFP biogenesis gene expression, we explored the relationship between cAMP, the Chp system and TFP regulation. We discovered that the Chp system controls TFP production through modulation of cAMP while control of TFP-dependent twitching motility is cAMP-independent. Overall, our data define a novel function for a chemotaxis-like system in controlling cAMP production and establish a regulatory link between the Chp system, TFP and other cAMP-dependent virulence systems.
Figures





References
-
- Ahn KS, Ha U, Jia J, Wu D, Jin S. The truA gene of Pseudomonas aeruginosa is required for the expression of type III secretory genes. Microbiology. 2004;150:539–547. - PubMed
-
- Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, Torres A. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162:1849–1858. - PubMed
-
- Baker DA, Kelly JM. Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol Microbiol. 2004;52:1229–1242. - PubMed
-
- Barker AP, Vasil AI, Filloux A, Ball G, Wilderman PJ, Vasil ML. A novel extracellular phospholipase C of Pseudomonas aeruginosa is required for phospholipid chemotaxis. Mol Microbiol. 2004;53:1089–1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

