Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity
- PMID: 20345918
- PMCID: PMC3036165
- DOI: 10.1111/j.1460-9568.2010.07156.x
Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity
Abstract
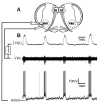
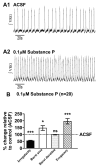
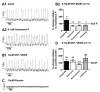
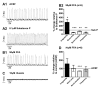
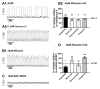
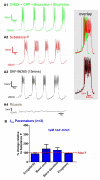
Neuromodulators, such as substance P (SubP), play an important role in modulating many rhythmic activities driven by central pattern generators (e.g. locomotion, respiration). However, the mechanism by which SubP enhances breathing regularity has not been determined. Here, we used mouse brainstem slices containing the pre-Bötzinger complex to demonstrate, for the first time, that SubP activates transient receptor protein canonical (TRPC) channels to enhance respiratory rhythm regularity. Moreover, SubP enhancement of network regularity is accomplished via selective enhancement of ICAN (inward non-specific cation current)-dependent intrinsic bursting properties. In contrast to INaP (persistent sodium current)-dependent pacemakers, ICAN-dependent pacemaker bursting activity is TRPC-dependent. Western Blots reveal TRPC3 and TRPC7 channels are expressed in rhythmically active ventral respiratory group island preparations. Taken together, these data suggest that SubP-mediated activation of TRPC3/7 channels underlies rhythmic ICAN-dependent pacemaker activity and enhances the regularity of respiratory rhythm activity.
Figures




References
-
- Barthe JY, Clarac F. Modulation of the spinal network for locomotion by substance P in the neonatal rat. Experimental Brain Research. 1997;115:485–492. - PubMed
-
- Boesmans W, Gomes P, Janssens J, Tack J, Berghe PV. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut. 2008;57:314–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

