Critical role of nitric oxide on nicotine-induced hyperactivation of dopaminergic nigrostriatal system: Electrophysiological and neurochemical evidence in rats
- PMID: 20345972
- PMCID: PMC6493862
- DOI: 10.1111/j.1755-5949.2010.00136.x
Critical role of nitric oxide on nicotine-induced hyperactivation of dopaminergic nigrostriatal system: Electrophysiological and neurochemical evidence in rats
Abstract
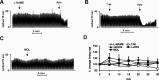
Nicotine, the main psychoactive ingredient in tobacco, stimulates dopamine (DA) function, increasing DA neuronal activity and DA release. DA is involved in both motor control and in the rewarding and reinforcing effects of nicotine; however, the complete understanding of its molecular mechanisms is yet to be attained. Substantial evidence indicates that the reinforcing properties of drugs of abuse, including nicotine, can be affected by the nitric oxide (NO) system, which may act by modulating central dopaminergic function. In this study, using single cell recordings in vivo coupled with microiontophoresis and microdialysis in freely moving animals, the role of NO signaling on the hyperactivation elicited by nicotine of the nigrostriatal system was investigated in rats. Nicotine induced a dose-dependent increase of the firing activity of the substantia nigra pars compacta (SNc) DA neurons and DA and 3,4-dihydroxyphenylacetic acid (DOPAC) release in the striatum. Pharmacological manipulation of the NO system did not produce any change under basal condition in terms of neuronal discharge and DA release. In contrast, pretreatments with two NO synthase (NOS) inhibitors, N-omega-nitro-l-arginine methyl ester (l-NAME) and 7-nitroindazole (7-NI) were both capable of blocking the nicotine-induced increase of SNc DA neuron activity and DA striatal levels. The effects of nicotine in l-NAME and 7-NI-pretreated rats were partially restored when rats were pretreated with the NO donor molsidomine. These results further support the evidence of an important role played by NO on modulation of dopaminergic function and drug addiction, thus revealing new pharmacological possibilities in the treatment of nicotine dependence and other DA dysfunctions.
Conflict of interest statement
All the authors do not have any conflict of interest.
Figures



References
-
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self‐administration. Psychopharmacology 1991;104:171–176. - PubMed
-
- Rose JE, Corrigall WA. Nicotine self‐administration in animals and humans: Similarities and differences. Psychopharmacology 1997;130:28–40. - PubMed
-
- Laviolette SR, Van Der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry 2003;8:50–59. - PubMed
-
- Clarke PBS, Fu DS, Jakubovic A, Fibiger HC. Evidence that Mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther 1988;246:701–708. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

