Predictors of additional intraocular pressure reduction in patients changed to latanoprost/timolol fixed combination
- PMID: 20346127
- PMCID: PMC2861019
- DOI: 10.1186/1471-2415-10-10
Predictors of additional intraocular pressure reduction in patients changed to latanoprost/timolol fixed combination
Abstract
Background: Given the growing number of ocular hypotensive medications available, it is important to be able to predict a positive response to therapy. The purpose of the present study was to identify predictors of an additional 10% intraocular pressure (IOP) reduction after 12 weeks of treatment with latanoprost/timolol fixed combination (FC) in patients requiring a change in their previous ocular hypotensive medication.
Methods: This multicenter, open-label, prospective, phase IIIb study included subjects >or=18 years of age with open-angle glaucoma (OAG) or ocular hypertension (OHT). Eligible subjects had baseline IOP >or=21 mmHg and insufficient response to current beta-blocker monotherapy. The primary efficacy analysis (logistic regression) identified predictors of a positive response after 12 weeks of latanoprost/timolol FC.
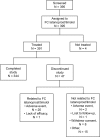
Results: The intent-to-treat (ITT) population included 383 subjects treated with >or=1 drop of FC and having >or=1 follow-up IOP assessment. Mean IOP was 22.19 +/- 2.16 mmHg at baseline and was reduced by 5.42 +/- 2.71 mmHg at study end. In all, 325 (84.9%) subjects had a positive response to latanoprost/timolol FC; the response rate was similar across groups: OAG (n = 208; 82.7%); OHT (n = 161; 87.6%); OAG+OHT (n = 14; 85.7%). Higher baseline IOP (odds ratio: 1.284; 95% confidence interval [CI]: 1.101, 1.497; p = 0.0014) and absence of adverse events (odds ratio: 0.318; 95% CI: 0.161, 0.629; p = 0.0010) were significant predictors of positive response. Age, gender, ethnic origin, diagnosis, family history of OAG/OHT, corneal thickness, and concomitant systemic beta-blocker were not significant predictors of a positive response in the ITT analysis. The FC was well tolerated. The most common adverse events were related to the eye and were consistent with known adverse events associated with latanoprost and timolol.
Conclusions: These results support the use of latanoprost/timolol FC in patients whose IOP is insufficiently controlled on beta-blocker monotherapy. Patients with higher baseline IOP levels and who do not experience adverse events while on therapy are most likely to achieve a positive response to latanoprost/timolol FC.
Trial registration: ClinicalTrials.gov NCT00230763.
Figures
Similar articles
-
A 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertension.Br J Ophthalmol. 2004 Feb;88(2):199-203. doi: 10.1136/bjo.2003.018234. Br J Ophthalmol. 2004. PMID: 14736774 Free PMC article. Clinical Trial.
-
Comparison of latanoprost with fixed-combination dorzolamide and timolol in adult patients with elevated intraocular pressure: an eight-week, randomized, open-label, parallel-group, multicenter study in Latin America.Clin Ther. 2004 May;26(5):755-68. doi: 10.1016/s0149-2918(04)90075-6. Clin Ther. 2004. PMID: 15220019 Clinical Trial.
-
Switching to Preservative-Free Tafluprost/Timolol Fixed-Dose Combination in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: Subanalysis of Data from the VISIONARY Study According to Baseline Monotherapy Treatment.Adv Ther. 2022 Aug;39(8):3501-3521. doi: 10.1007/s12325-022-02166-6. Epub 2022 May 7. Adv Ther. 2022. PMID: 35524840 Free PMC article.
-
Latanoprost : an update of its use in glaucoma and ocular hypertension.Drugs Aging. 2003;20(8):597-630. doi: 10.2165/00002512-200320080-00005. Drugs Aging. 2003. PMID: 12795627 Review.
-
An evaluation of the fixed-combination of latanoprost and timolol for use in open-angle glaucoma and ocular hypertension.Expert Opin Pharmacother. 2004 Apr;5(4):909-21. doi: 10.1517/14656566.5.4.909. Expert Opin Pharmacother. 2004. PMID: 15102573 Review.
Cited by
-
Current primary open-angle glaucoma treatments and future directions.Clin Ophthalmol. 2012;6:1699-707. doi: 10.2147/OPTH.S32933. Epub 2012 Oct 23. Clin Ophthalmol. 2012. PMID: 23118520 Free PMC article.
-
The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy.Br J Ophthalmol. 2012 Feb;96(2):254-7. doi: 10.1136/bjo.2010.196899. Epub 2011 Mar 24. Br J Ophthalmol. 2012. PMID: 21436180 Free PMC article.
-
Effect of latanoprost/timolol and dorzolamide/tiomolol on intraocular pressure after phacoemulsification surgery.Int J Ophthalmol. 2011;4(2):190-4. doi: 10.3980/j.issn.2222-3959.2011.02.17. Epub 2011 Apr 18. Int J Ophthalmol. 2011. PMID: 22553640 Free PMC article.
References
-
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. - PubMed
-
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical