Chloride channels and transporters in human corneal epithelium
- PMID: 20346358
- PMCID: PMC2873124
- DOI: 10.1016/j.exer.2010.03.013
Chloride channels and transporters in human corneal epithelium
Abstract
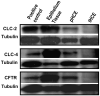
Transport of water and electrolytes is critical for corneal clarity. Recent studies indicate another important function of transport of ions and electrolytes - establishing wound electric fields that guide cell migration. We found chloride (Cl(-)) flux is a major component of the corneal wound electric current. In order to elucidate the mechanisms of Cl(-) transport, we studied Cl(-) channels and transporters in human corneal epithelial (HCE) cells. We tested a transformed human corneal epithelial cell line (tHCE), primary cultures of human corneal epithelial cells (pHCE), and human donor corneas. We first used RT-PCR to determine expression levels of mRNA of CLC (Cl(-) channels/transporters of CLC gene family) family members and CFTR (cystic fibrosis transmembrane conductance regulator) in HCE cells. We then confirmed protein expression and distribution of selected CLC family members and CFTR with Western blot and immunofluorescence confocal microscopy. Finally, Cl(-) currents were recorded with electrophysiological techniques. The mRNAs of CLC-2, CLC-3, CLC-4, CLC-5, CLC-6, and CFTR were detected in the HCE cell line. CLC-1 and CLC-7 were not detectable. Western blot and immunostaining confirmed protein expression and distribution of CLC-2, CLC-3, CLC-4, CLC-6 and CFTR in human corneal epithelium. CLC-2 preferentially labeled the apical and basal layers, while CLC-3 and CLC-4 labeled only the superficial layer. CLC-6 and CFTR labeling showed a unique gradient with strong staining in apical layers which gradually decreased towards the basal layers. Corneal endothelium was positive for CLC-2, CLC-3, CLC-4, CLC-6 and possibly CFTR. Human corneal epithelial cells demonstrated voltage dependent Cl(-) currents. HCE cells express functional Cl(-) channels and transporters. CLC-2, CLC-3, CLC-4, CLC-6, and CFTR had distinct expression patterns in human corneal epithelium. Those molecules and their distribution may play important roles in maintaining resting Cl(-) fluxes and in regulating Cl(-) flux at corneal wounds, which may be a major contributor to wound electrical signaling.
Published by Elsevier Ltd.
Figures





Similar articles
-
Studies on the expression of mRNA for anion transport related proteins in corneal endothelial cells.Curr Eye Res. 2001 Jan;22(1):1-7. doi: 10.1076/ceyr.22.1.1.6981. Curr Eye Res. 2001. PMID: 11402373
-
Chloride channel gene expression in the rabbit cornea.Mol Vis. 2004 Dec 30;10:1028-37. Mol Vis. 2004. PMID: 15635293
-
SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents.Am J Physiol Cell Physiol. 2004 Nov;287(5):C1173-83. doi: 10.1152/ajpcell.00528.2003. Epub 2004 Jun 22. Am J Physiol Cell Physiol. 2004. PMID: 15213059
-
CLC-0 and CFTR: chloride channels evolved from transporters.Physiol Rev. 2008 Apr;88(2):351-87. doi: 10.1152/physrev.00058.2006. Physiol Rev. 2008. PMID: 18391167 Review.
-
Expression and function of CLC and cystic fibrosis transmembrane conductance regulator chloride channels in renal epithelial tubule cells: pathophysiological implications.Chang Gung Med J. 2007 Jan-Feb;30(1):17-25. Chang Gung Med J. 2007. PMID: 17477025 Review.
Cited by
-
Making Sense of Electrical Stimulation: A Meta-analysis for Wound Healing.Ann Biomed Eng. 2024 Feb;52(2):153-177. doi: 10.1007/s10439-023-03371-2. Epub 2023 Sep 24. Ann Biomed Eng. 2024. PMID: 37743460 Free PMC article. Review.
-
Ionic components of electric current at rat corneal wounds.PLoS One. 2011 Feb 25;6(2):e17411. doi: 10.1371/journal.pone.0017411. PLoS One. 2011. PMID: 21364900 Free PMC article.
-
The quantification of zebrafish ocular-associated proteins provides hints for sex-biased visual impairments and perception.Heliyon. 2024 Jun 13;10(12):e33057. doi: 10.1016/j.heliyon.2024.e33057. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38994070 Free PMC article.
-
Novel 5-substituted benzyloxy-2-arylbenzofuran-3-carboxylic acids as calcium activated chloride channel inhibitors.Bioorg Med Chem. 2012 Jul 15;20(14):4237-44. doi: 10.1016/j.bmc.2012.05.074. Epub 2012 Jun 6. Bioorg Med Chem. 2012. PMID: 22739085 Free PMC article.
-
Sulfur mustard corneal injury is associated with alterations in the epithelial basement membrane and stromal extracellular matrix.Exp Mol Pathol. 2022 Oct;128:104807. doi: 10.1016/j.yexmp.2022.104807. Epub 2022 Jul 4. Exp Mol Pathol. 2022. PMID: 35798063 Free PMC article.
References
-
- Al-Nakkash L, Iserovich P, Coca-Prados M, Yang H, Reinach PS. Functional and molecular characterization of a volume-activated chloride channel in rabbit corneal epithelial cells. The Journal of membrane biology. 2004;201:41–49. - PubMed
-
- Al-Nakkash L, Reinach PS. Activation of a CFTR-mediated chloride current in a rabbit corneal epithelial cell line. Investigative ophthalmology & visual science. 2001;42:2364–2370. - PubMed
-
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. - PubMed
-
- Barker AT, Jaffe LF, Vanable JW., Jr The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242:R358–366. - PubMed
-
- Bonanno JA, Srinivas SP. Cyclic AMP activates anion channels in cultured bovine corneal endothelial cells. Experimental eye research. 1997;64:953–962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

