A defined long-term in vitro tissue engineered model of neuromuscular junctions
- PMID: 20346499
- PMCID: PMC2925240
- DOI: 10.1016/j.biomaterials.2010.02.055
A defined long-term in vitro tissue engineered model of neuromuscular junctions
Abstract
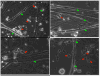
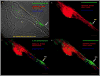
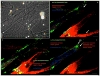
Neuromuscular junction (NMJ) formation, occurring between motoneurons and skeletal muscle, is a complex multistep process involving a variety of signaling molecules and pathways. In vitro motoneuron-muscle co-cultures are powerful tools to study the role of different growth factors, hormones and cellular structures involved in NMJ formation. In this study, a serum-free culture system utilizing defined temporal growth factor application and a non-biological substrate resulted in the formation of robust NMJs. The system resulted in long-term survival of the co-culture and selective expression of neonatal myosin heavy chain, a marker of myotube maturation. NMJ formation was verified by colocalization of dense clusters of acetylcholine receptors visualized using alpha-bungarotoxin and synaptophysin containing vesicles present in motoneuron axonal terminals. This model will find applications in basic NMJ research and tissue engineering applications such as bio-hybrid device development for limb prosthesis and regenerative medicine as well as for high-throughput drug and toxin screening applications.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Witzemann V. Development of the neuromuscular junction. Cell Tissue Res. 2006;326(2):263–71. - PubMed
-
- Colomar A, Robitaille R. Glial modulation of synaptic transmission at the neuromuscular junction. Glia. 2004;47(3):284–9. - PubMed
-
- English AW. Cytokines, growth factors and sprouting at the neuromuscular junction. J Neurocytol. 2003;32(5):943–60. - PubMed
-
- Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, et al. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane- modified surfaces. J Am Chem Soc. 1998;120(47):12169–77.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

