Gel mobilities of linking-number topoisomers and their dependence on DNA helical repeat and elasticity
- PMID: 20346570
- PMCID: PMC2867096
- DOI: 10.1016/j.bpc.2010.02.016
Gel mobilities of linking-number topoisomers and their dependence on DNA helical repeat and elasticity
Abstract
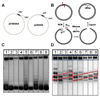
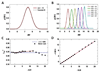
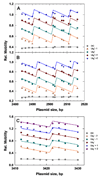
Agarose-gel electrophoresis has been used for more than thirty years to characterize the linking-number (Lk) distribution of closed-circular DNA molecules. Although the physical basis of this technique remains poorly understood, the gel-electrophoretic behavior of covalently closed DNAs has been used to determine the local unwinding of DNA by proteins and small-molecule ligands, characterize supercoiling-dependent conformational transitions in duplex DNA, and to measure helical-repeat changes due to shifts in temperature and ionic strength. Those results have been analyzed by assuming that the absolute mobility of a particular topoisomer is mainly a function of the integral number of superhelical turns, and thus a slowly varying function of plasmid molecular weight. In examining the mobilities of Lk topoisomers for a series of plasmids that differ incrementally in size over more than one helical turn, we found that the size-dependent agarose-gel mobility of individual topoisomers with identical values of Lk (but different values of the excess linking number, DeltaLk) vary dramatically over a duplex turn. Our results suggest that a simple semi-empirical relationship holds between the electrophoretic mobility of linking-number topoisomers and their average writhe in solution.
Figures

Similar articles
-
Electrophoretic mobility of supercoiled, catenated and knotted DNA molecules.Nucleic Acids Res. 2015 Feb 27;43(4):e24. doi: 10.1093/nar/gku1255. Epub 2014 Nov 20. Nucleic Acids Res. 2015. PMID: 25414338 Free PMC article.
-
Analysis of DNA topoisomers, knots, and catenanes by agarose gel electrophoresis.Methods Mol Biol. 2009;582:11-25. doi: 10.1007/978-1-60761-340-4_2. Methods Mol Biol. 2009. PMID: 19763938
-
Early melting of supercoiled DNA topoisomers observed by TGGE.Nucleic Acids Res. 2000 Jun 1;28(11):E51. doi: 10.1093/nar/28.11.e51. Nucleic Acids Res. 2000. PMID: 10871350 Free PMC article.
-
Temperature dependence of the gel electrophoretic mobility of superhelical DNA.Nucleic Acids Res. 1985 Mar 11;13(5):1665-82. doi: 10.1093/nar/13.5.1665. Nucleic Acids Res. 1985. PMID: 2987835 Free PMC article.
-
Migration properties of circular DNAs using orthogonal-field-alternation gel electrophoresis.Electrophoresis. 1989 May-Jun;10(5-6):283-90. doi: 10.1002/elps.1150100503. Electrophoresis. 1989. PMID: 2670543 Review.
Cited by
-
Deconvolution of nucleic-acid length distributions: a gel electrophoresis analysis tool and applications.Nucleic Acids Res. 2019 Sep 19;47(16):e92. doi: 10.1093/nar/gkz534. Nucleic Acids Res. 2019. PMID: 31226202 Free PMC article.
-
Suitability of double-stranded DNA as a molecular standard for the validation of analytical ultracentrifugation instruments.Eur Biophys J. 2023 Jul;52(4-5):267-280. doi: 10.1007/s00249-023-01671-y. Epub 2023 Jul 27. Eur Biophys J. 2023. PMID: 37501021 Free PMC article.
-
Dataset on the effects of spermidine on linking number differences between histone H1-free and histone H1-bound circular polynucleosomes.Data Brief. 2018 Feb 3;17:709-715. doi: 10.1016/j.dib.2018.01.091. eCollection 2018 Apr. Data Brief. 2018. PMID: 29511714 Free PMC article.
-
Electrophoretic mobility of supercoiled, catenated and knotted DNA molecules.Nucleic Acids Res. 2015 Feb 27;43(4):e24. doi: 10.1093/nar/gku1255. Epub 2014 Nov 20. Nucleic Acids Res. 2015. PMID: 25414338 Free PMC article.
-
Structural diversity of supercoiled DNA.Nat Commun. 2015 Oct 12;6:8440. doi: 10.1038/ncomms9440. Nat Commun. 2015. PMID: 26455586 Free PMC article.
References
-
- White JH, Gallo RM, Bauer WR. Closed circular DNA as a probe for protein-induced structural changes. Trends Biochem Sci. 1992;17:7–12. - PubMed
-
- Clendenning JB, Naimushin AN, Fujimoto BS, Stewart DW, Schurr JM. Effect of ethidium binding and superhelix density on the supercoiling free energy and torsion and bending constants of p30δ DNA. Biophys Chem. 1994;52:191–218. - PubMed
-
- Lilley DM, Chen D, Bowater RP. DNA supercoiling and transcription: Topological coupling of promoters. Q Rev Biophys. 1996;29:203–225. - PubMed
-
- Rybenkov VV, Ullsperger C, Vologodskii AV, Cozzarelli NR. Simplification of DNA topology below equilibrium values by type II topoisomerases. Science. 1997;277:690–693. - PubMed
-
- Wang JC. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974;89:783–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

