The molecular regulation of programmed necrotic cell injury
- PMID: 20346680
- PMCID: PMC2904865
- DOI: 10.1016/j.tibs.2010.03.001
The molecular regulation of programmed necrotic cell injury
Abstract
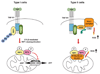
Proper regulation of cell death is essential for metazoan development and functions. Unlike apoptosis, necrosis is a more inflammatory form of cell death that might contribute to antiviral immunity. Indeed, necrotic cell injury is distinguished from apoptosis by extensive organelle and cell swelling and plasma membrane rupture. Recent evidence indicates that an elaborate biochemical network emanating from receptors in the TNF superfamily can induce apoptosis as well as necrotic cell death. The induction of necrosis by TNF-like cytokines requires biochemical components that are distinct from those involved in apoptosis. Specifically, serine/threonine protein kinases in the receptor interacting protein (RIP) family are required for "programmed" necrotic cell injury. In this review, we discuss the molecular crosstalk between apoptosis and programmed necrosis, with a special emphasis on how caspases, protein ubiquitylation and phosphorylation regulate the induction of necrotic cell injury.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

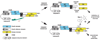
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

