CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development
- PMID: 20346773
- PMCID: PMC2865198
- DOI: 10.1016/j.immuni.2010.03.006
CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development
Abstract
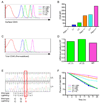
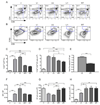
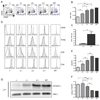
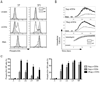
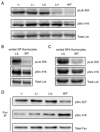
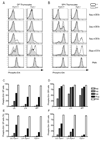
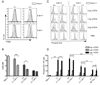
The kinase-phosphatase pair Csk and CD45 reciprocally regulate phosphorylation of the inhibitory tyrosine of the Src family kinases Lck and Fyn. T cell receptor (TCR) signaling and thymic development require CD45 expression but proceed constitutively in the absence of Csk. Here, we show that relative titration of CD45 and Csk expression reveals distinct regulation of basal and inducible TCR signaling during thymic development. Low CD45 expression is sufficient to rescue inducible TCR signaling and positive selection, whereas high expression is required to reconstitute basal TCR signaling and beta selection. CD45 has a dual positive and negative regulatory role during inducible but not basal TCR signaling. By contrast, Csk titration regulates basal but not inducible signaling. High physiologic expression of CD45 is thus required for two reasons-to downmodulate inducible TCR signaling during positive selection and to counteract Csk during basal TCR signaling.
Figures
Similar articles
-
Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling.Sci Signal. 2011 Sep 13;4(190):ra59. doi: 10.1126/scisignal.2001893. Sci Signal. 2011. PMID: 21917715 Free PMC article.
-
Autonomous maturation of alpha/beta T lineage cells in the absence of COOH-terminal Src kinase (Csk).J Exp Med. 2001 Apr 2;193(7):815-26. doi: 10.1084/jem.193.7.815. J Exp Med. 2001. PMID: 11283154 Free PMC article.
-
Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases.Mol Cell Biol. 1993 Mar;13(3):1651-6. doi: 10.1128/mcb.13.3.1651-1656.1993. Mol Cell Biol. 1993. PMID: 8441403 Free PMC article.
-
New insights into the transmembrane protein tyrosine phosphatase CD45.Int J Biochem Cell Biol. 2001 Nov;33(11):1041-6. doi: 10.1016/s1357-2725(01)00075-9. Int J Biochem Cell Biol. 2001. PMID: 11551820 Review.
-
Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation.Oncogene. 2004 Oct 18;23(48):7990-8000. doi: 10.1038/sj.onc.1208074. Oncogene. 2004. PMID: 15489916 Review.
Cited by
-
Protein Tyrosine Phosphatase CD45 As an Immunity Regulator and a Potential Effector of CAR-T therapy.Acta Naturae. 2023 Jul-Sep;15(3):17-26. doi: 10.32607/actanaturae.25438. Acta Naturae. 2023. PMID: 37908772 Free PMC article.
-
CD28 sensitizes TCR Ca²⁺ signaling during Ag-independent polarization of plasma membrane rafts.J Immunol. 2013 Sep 15;191(6):3073-81. doi: 10.4049/jimmunol.1300485. Epub 2013 Aug 21. J Immunol. 2013. PMID: 23966623 Free PMC article.
-
T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition.Science. 2017 Mar 31;355(6332):1428-1433. doi: 10.1126/science.aaf1292. Epub 2017 Mar 9. Science. 2017. PMID: 28280247 Free PMC article.
-
Targeting Protein Tyrosine Phosphatases to Improve Cancer Immunotherapies.Cells. 2024 Jan 25;13(3):231. doi: 10.3390/cells13030231. Cells. 2024. PMID: 38334623 Free PMC article. Review.
-
TCR Signaling: Mechanisms of Initiation and Propagation.Trends Biochem Sci. 2018 Feb;43(2):108-123. doi: 10.1016/j.tibs.2017.11.008. Epub 2017 Dec 18. Trends Biochem Sci. 2018. PMID: 29269020 Free PMC article. Review.
References
-
- Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. - PubMed
-
- Ashwell JD, D'Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–416. - PubMed
-
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. - PubMed
-
- Becker AM, DeFord-Watts LM, Wuelfing C, van Oers NS. The constitutive tyrosine phosphorylation of CD3zeta results from TCR-MHC interactions that are independent of thymic selection. J Immunol. 2007;178:4120–4128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

