An optimally constrained V3 peptide is a better immunogen than its linear homolog or HIV-1 gp120
- PMID: 20347111
- PMCID: PMC2862131
- DOI: 10.1016/j.virol.2010.03.007
An optimally constrained V3 peptide is a better immunogen than its linear homolog or HIV-1 gp120
Abstract
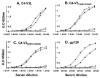
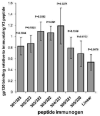
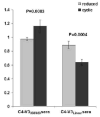
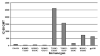
Synthetic peptides offer an attractive option for development of a V3-directed vaccine. However, immunization with flexible linear peptides may result in an immune response to multiple conformations, many of which differ from the native conformation of the corresponding region in the protein. Here we show that optimization of the location of a disulfide bond in peptides constrained to mimic the beta-hairpin conformation of the V3, yields an immunogen that elicits a 30-fold stronger HIV-1 neutralizing response in rabbits compared with the homologous linear V3 peptide. The HIV-1 neutralizing response elicited by the optimally constrained peptide is also significantly stronger than that elicited by a gp120 construct in which the V3 is exposed. Neutralization of an HIV-1 strain that shares only 72% identity with the immunizing peptide was demonstrated. The most effective immunogen was also able to neutralize primary isolates that are more resistant to neutralization such as SS1196 and 6535.
2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
HIV-1 peptide vaccine candidates: selecting constrained V3 peptides with highest affinity to antibody 447-52D.Biochemistry. 2009 Aug 25;48(33):7867-77. doi: 10.1021/bi900146g. Biochemistry. 2009. PMID: 19552398
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Synergism between a CD4-mimic peptide and antibodies elicited by a constrained V3 peptide.AIDS Res Hum Retroviruses. 2013 Apr;29(4):718-24. doi: 10.1089/AID.2012.0189. Epub 2012 Dec 26. AIDS Res Hum Retroviruses. 2013. PMID: 23176398 Free PMC article.
-
Elicitation of broadly reactive antibodies against glycan-modulated neutralizing V3 epitopes of HIV-1 by immune complex vaccines.Vaccine. 2013 Nov 4;31(46):5413-21. doi: 10.1016/j.vaccine.2013.09.010. Epub 2013 Sep 16. Vaccine. 2013. PMID: 24051158 Free PMC article.
-
Induction of Heterologous Tier 2 HIV-1-Neutralizing and Cross-Reactive V1/V2-Specific Antibodies in Rabbits by Prime-Boost Immunization.J Virol. 2016 Sep 12;90(19):8644-60. doi: 10.1128/JVI.00853-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440894 Free PMC article.
Cited by
-
Identification of Peptide Mimics of a Glycan Epitope on the Surface of Parasitic Nematode Larvae.PLoS One. 2016 Aug 31;11(8):e0162016. doi: 10.1371/journal.pone.0162016. eCollection 2016. PLoS One. 2016. PMID: 27579674 Free PMC article.
-
Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from HIV-1-infected individuals.Virology. 2012 Jun 5;427(2):198-207. doi: 10.1016/j.virol.2012.02.003. Epub 2012 Mar 7. Virology. 2012. PMID: 22402248 Free PMC article.
-
Peptides to combat viral infectious diseases.Peptides. 2020 Dec;134:170402. doi: 10.1016/j.peptides.2020.170402. Epub 2020 Sep 1. Peptides. 2020. PMID: 32889022 Free PMC article. Review.
-
The C4 region as a target for HIV entry inhibitors--NMR mapping of the interacting segments of T20 and gp120.FEBS J. 2015 Dec;282(24):4643-57. doi: 10.1111/febs.13541. Epub 2015 Oct 22. FEBS J. 2015. PMID: 26432362 Free PMC article.
-
Induction of neutralizing antibodies in rhesus macaques using V3 mimotope peptides.Vaccine. 2016 May 23;34(24):2713-21. doi: 10.1016/j.vaccine.2016.04.027. Epub 2016 Apr 19. Vaccine. 2016. PMID: 27102818 Free PMC article.
References
-
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–13252. - PMC - PubMed
-
- Bryder K, Sbai H, Nielsen HV, Corbet S, Nielsen C, Whalen RG, Fomsgaard A. Improved immunogenicity of HIV-1 epitopes in HBsAg chimeric DNA vaccine plasmids by structural mutations of HBsAg. DNA Cell Biol. 1999;18(3):219–225. - PubMed
-
- Cabezas E, Wang M, Parren PW, Stanfield RL, Satterthwait AC. A structure-based approach to a synthetic vaccine for HIV-1. Biochemistry. 2000;39(47):14377–14391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

