The role of a recombinant fragment of laminin-332 in integrin alpha3beta1-dependent cell binding, spreading and migration
- PMID: 20347131
- PMCID: PMC2861493
- DOI: 10.1016/j.biomaterials.2010.03.003
The role of a recombinant fragment of laminin-332 in integrin alpha3beta1-dependent cell binding, spreading and migration
Abstract
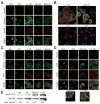
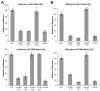
The extracellular matrix (ECM) is thought to be an essential component of tissue scaffolding and engineering because it fulfills fundamental functions related to cell adhesion, migration, and three-dimensional organization. Natural ECM preparations, however, are challenging to work with because they are comprised of macromolecules that are large and insoluble in their functional state. Functional fragments of ECM macromolecules are a viable answer to this challenge, as demonstrated by the RGD-based engineered scaffolds, where the tri-peptide, Arg-Gly-Asp (RGD), represents the minimal functional unit of fibronectin and related ECM. Laminins (Ln) are main components of epithelial tissues, since they enter into the composition of basement membranes. Application of Ln to epithelial tissue engineering would be desirable, since they could help mimic ideal functional conditions for both lining and glandular epithelial tissues. However, functional fragments of Ln that could be used in artificial settings have not been characterized in detail. In this paper, we describe the production and application of the recombinant LG4 (rLG4) fragment of laminin-332 (Ln-332), and show that it mimics three fundamental functional properties of Ln-332: integrin-mediated cell adhesion, spreading, and migration. Adhesive structures formed by cells on rLG4 closely resemble those formed on Ln-332, as judged by microscopy-based analyses of their molecular composition. As on Ln-332, focal adhesion kinase (FAK) is phosphorylated in cells adhering to rLG4, and colocalized with other focal adhesion components. We conclude that rLG4 could be a useful substitute to recapitulate, in vitro, the tissue scaffolding properties of Ln-332.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


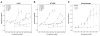



Similar articles
-
The LG3 module of laminin-5 harbors a binding site for integrin alpha3beta1 that promotes cell adhesion, spreading, and migration.J Biol Chem. 2001 Aug 31;276(35):33045-53. doi: 10.1074/jbc.M100798200. Epub 2001 Jun 6. J Biol Chem. 2001. PMID: 11395486
-
Laminin-332-beta1 integrin interactions negatively regulate invadopodia.J Cell Physiol. 2010 Apr;223(1):134-42. doi: 10.1002/jcp.22018. J Cell Physiol. 2010. PMID: 20039268 Free PMC article.
-
The PPFLMLLKGSTR motif in globular domain 3 of the human laminin-5 alpha3 chain is crucial for integrin alpha3beta1 binding and cell adhesion.Exp Cell Res. 2005 Mar 10;304(1):317-27. doi: 10.1016/j.yexcr.2004.11.009. Epub 2004 Dec 8. Exp Cell Res. 2005. PMID: 15707596
-
Laminins in osteogenic differentiation and pluripotency maintenance.Differentiation. 2020 Jul-Aug;114:13-19. doi: 10.1016/j.diff.2020.05.002. Epub 2020 May 16. Differentiation. 2020. PMID: 32473527 Review.
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
Cited by
-
Slc15a1 is involved in the transport of synthetic F5-peptide into the seminiferous epithelium in adult rat testes.Sci Rep. 2015 Nov 5;5:16271. doi: 10.1038/srep16271. Sci Rep. 2015. PMID: 26537751 Free PMC article.
-
Laminin-332 cleavage by matriptase alters motility parameters of prostate cancer cells.Prostate. 2011 Feb 1;71(2):184-96. doi: 10.1002/pros.21233. Prostate. 2011. PMID: 20672321 Free PMC article.
-
Surfce Functionalized via AdLAMA3 Multilayer Coating for Re-epithelization Around Titanium Implants.Front Bioeng Biotechnol. 2020 Jun 11;8:624. doi: 10.3389/fbioe.2020.00624. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32596232 Free PMC article.
-
Overexpression of α3, β3 and γ2 chains of laminin-332 is associated with poor prognosis in pancreatic ductal adenocarcinoma.Oncol Lett. 2018 Jul;16(1):199-210. doi: 10.3892/ol.2018.8678. Epub 2018 May 9. Oncol Lett. 2018. PMID: 29928402 Free PMC article.
-
Adenovirus-Mediated LAMA3 Transduction Enhances Hemidesmosome Formation and Periodontal Reattachment during Wound Healing.Mol Ther Methods Clin Dev. 2020 Jun 4;18:291-303. doi: 10.1016/j.omtm.2020.06.001. eCollection 2020 Sep 11. Mol Ther Methods Clin Dev. 2020. PMID: 32671133 Free PMC article.
References
-
- Menko AS, Boettinger D. Occupation of the extracellular matrix receptor integrin is a control point for differentiation. Cell. 1987;51:51–7. - PubMed
-
- Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;119(2):174–80. - PubMed
-
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

