Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice
- PMID: 20347173
- PMCID: PMC2877801
- DOI: 10.1016/j.jhep.2009.10.037
Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice
Abstract
Background & aims: Suppression of the hypothalamic-pituitary-adrenal axis occurs in cirrhosis and cholestasis and is associated with increased concentrations of bile acids. We investigated whether this was mediated through bile acids acting to impair steroid clearance by inhibiting glucocorticoid metabolism by 5beta-reductase.
Methods: The effect of bile acids on glucocorticoid metabolism was studied in vitro in hepatic subcellular fractions and hepatoma cells, allowing quantitation of the kinetics and transcript abundance of 5beta-reductase. Metabolism was subsequently examined in vivo in rats following dietary manipulation or bile duct ligation. Finally, glucocorticoid metabolism was assessed in humans with obstructive jaundice.
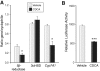
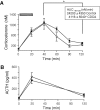
Results: In rat hepatic cytosol, chenodeoxycholic acid competitively inhibited 5beta-reductase (K(i) 9.19+/-0.40 microM) and reduced its transcript abundance (in H4iiE cells) and promoter activity (reporter system, HepG2 cells). In Wistar rats, dietary chenodeoxycholic acid (1% w/w chow) inhibited hepatic 5beta-reductase activity, reduced urinary excretion of 3alpha,5beta-tetrahydrocorticosterone and reduced adrenal weight. Conversely, a fat-free diet suppressed bile acid levels and increased hepatic 5beta-reductase activity, supplementation of the fat-free diet with CDCA reduced 5beta-reductase activity, and urinary 3alpha,5beta-reduced corticosterone. Cholestasis in rats suppressed hepatic 5beta-reductase activity and transcript abundance. In eight women with obstructive jaundice, relative urinary excretion of 3alpha,5beta-tetrahydrocortisol was significantly lower than in healthy controls.
Conclusion: These data suggest a novel role for bile acids in inhibiting hepatic glucocorticoid clearance, of sufficient magnitude to suppress hypothalamic-pituitary-adrenal axis activity. Elevated hepatic bile acids may account for adrenal insufficiency in liver disease.
Crown Copyright (c) 2010. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Relative adrenal insufficiency in mice deficient in 5α-reductase 1.J Endocrinol. 2014 Aug;222(2):257-66. doi: 10.1530/JOE-13-0563. Epub 2014 May 28. J Endocrinol. 2014. PMID: 24872577 Free PMC article.
-
Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high-fat feeding in Wistar rats.Endocrinology. 2005 Feb;146(2):913-9. doi: 10.1210/en.2004-1063. Epub 2004 Nov 18. Endocrinology. 2005. PMID: 15550507
-
Suppression of the HPA Axis During Cholestasis Can Be Attributed to Hypothalamic Bile Acid Signaling.Mol Endocrinol. 2015 Dec;29(12):1720-30. doi: 10.1210/me.2015-1087. Epub 2015 Oct 2. Mol Endocrinol. 2015. PMID: 26431088 Free PMC article.
-
Hypothalamic-pituitary-adrenal-glucocorticoid axis function in rheumatoid arthritis.J Rheumatol. 1996 Apr;23(4):577-81. J Rheumatol. 1996. PMID: 8730107 Review. No abstract available.
-
[Steroid therapy and adrenal function].Recenti Prog Med. 2000 Nov;91(11):594-600. Recenti Prog Med. 2000. PMID: 11125954 Review. Italian.
Cited by
-
Prevention of gallstones by Lidan Granule: Insight into underlying mechanisms using a guinea pig model.Biomed Rep. 2016 Jul;5(1):50-56. doi: 10.3892/br.2016.672. Epub 2016 May 6. Biomed Rep. 2016. PMID: 27347405 Free PMC article.
-
An Analysis of the Hypothalamic-Pituitary-Adrenal Axis Functions in Cirrhotic Rats in Response to Surgical Stress.Surg Res Pract. 2018 Jun 28;2018:7606304. doi: 10.1155/2018/7606304. eCollection 2018. Surg Res Pract. 2018. PMID: 30050969 Free PMC article.
-
Cholestasis-induced bile acid elevates estrogen level via farnesoid X receptor-mediated suppression of the estrogen sulfotransferase SULT1E1.J Biol Chem. 2018 Aug 17;293(33):12759-12769. doi: 10.1074/jbc.RA118.001789. Epub 2018 Jun 21. J Biol Chem. 2018. PMID: 29929982 Free PMC article.
-
Bile acids increase steroidogenesis in cholemic mice and induce cortisol secretion in adrenocortical H295R cells via S1PR2, ERK and SF-1.Liver Int. 2019 Nov;39(11):2112-2123. doi: 10.1111/liv.14052. Epub 2019 Feb 17. Liver Int. 2019. PMID: 30664326 Free PMC article.
-
Maternal Glucocorticoid Metabolism Across Pregnancy: A Potential Mechanism Underlying Fetal Glucocorticoid Exposure.J Clin Endocrinol Metab. 2020 Mar 1;105(3):e782-90. doi: 10.1210/clinem/dgz313. J Clin Endocrinol Metab. 2020. PMID: 32108902 Free PMC article.
References
-
- Harry R., Auzinger G., Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology. 2002;36:395–402. - PubMed
-
- Tsai M.H., Peng Y.S., Chen Y.C., Liu N.J., Ho Y.P., Fang J.T. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43:673–681. - PubMed
-
- Fernandez J., Escorsell A., Zabalza M., Felipe V., Navasa M., Mas A. Adrenal insufficiency in patients with cirrhosis and septic shock. Hepatology. 2006;44:1288–1295. - PubMed
-
- Demelia L., Solinas A., Poma R., Vallebona E., Pitzus F. Hypothalamo–pituitary–adrenal function in liver cirrhosis of viral etiology. Ann Ital Med Int. 1991;6:203–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

