Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort
- PMID: 20347483
- PMCID: PMC2858802
- DOI: 10.1016/S0140-6736(10)60057-8
Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort
Abstract
Background: Co-trimoxazole prophylaxis can reduce mortality from untreated HIV infection in Africa; whether benefits occur alongside combination antiretroviral therapy (ART) is unclear. We estimated the effect of prophylaxis after ART initiation in adults.
Methods: Participants in our observational analysis were from the DART randomised trial of management strategies in HIV-infected, symptomatic, previously untreated African adults starting triple-drug ART with CD4 counts lower than 200 cells per muL. Co-trimoxazole prophylaxis was not routinely used or randomly allocated, but was variably prescribed by clinicians. We estimated effects on clinical outcomes, CD4 cell count, and body-mass index (BMI) using marginal structural models to adjust for time-dependent confounding by indication. DART was registered, number ISRCTN13968779.
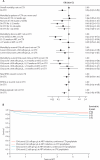
Findings: 3179 participants contributed 14 214 years of follow-up (8128 [57%] person-years on co-trimoxazole). Time-dependent predictors of co-trimoxazole use were current CD4 cell count, haemoglobin concentration, BMI, and previous WHO stage 3 or 4 events on ART. Present prophylaxis significantly reduced mortality (odds ratio 0.65, 95% CI 0.50-0.85; p=0.001). Mortality risk reduction on ART was substantial to 12 weeks (0.41, 0.27-0.65), sustained from 12-72 weeks (0.56, 0.37-0.86), but not evident subsequently (0.96, 0.63-1.45; heterogeneity p=0.02). Variation in mortality reduction was not accounted for by time on co-trimoxazole or current CD4 cell count. Prophylaxis reduced frequency of malaria (0.74, 0.63-0.88; p=0.0005), an effect that was maintained with time, but we observed no effect on new WHO stage 4 events (0.86, 0.69-1.07; p=0.17), CD4 cell count (difference vs non-users, -3 cells per muL [-12 to 6]; p=0.50), or BMI (difference vs non-users, -0.04 kg/m(2) [-0.20 to 0.13); p=0.68].
Interpretation: Our results reinforce WHO guidelines and provide strong motivation for provision of co-trimoxazole prophylaxis for at least 72 weeks for all adults starting combination ART in Africa.
Funding: UK Medical Research Council, the UK Department for International Development, the Rockefeller Foundation, GlaxoSmithKline, Gilead Sciences, Boehringer-Ingelheim, and Abbott Laboratories.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Co-trimoxazole, cART, and non-AIDS infectious diseases.Lancet. 2010 Apr 10;375(9722):1231-3. doi: 10.1016/S0140-6736(10)60200-0. Epub 2010 Mar 27. Lancet. 2010. PMID: 20347482 No abstract available.
Similar articles
-
A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa.N Engl J Med. 2014 Jan 2;370(1):41-53. doi: 10.1056/NEJMoa1214901. N Engl J Med. 2014. PMID: 24382064 Free PMC article. Clinical Trial.
-
Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial.Lancet. 2010 Jan 9;375(9709):123-31. doi: 10.1016/S0140-6736(09)62067-5. Epub 2009 Dec 8. Lancet. 2010. PMID: 20004464 Free PMC article. Clinical Trial.
-
Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda.Lancet. 2004 Oct 16-22;364(9443):1428-34. doi: 10.1016/S0140-6736(04)17225-5. Lancet. 2004. PMID: 15488218 Clinical Trial.
-
The expanding role of co-trimoxazole in developing countries.Lancet Infect Dis. 2015 Mar;15(3):327-39. doi: 10.1016/S1473-3099(14)71011-4. Epub 2015 Jan 21. Lancet Infect Dis. 2015. PMID: 25618179 Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Self-care practices and experiences of people living with HIV not receiving antiretroviral therapy in an urban community of Lusaka, Zambia: implications for HIV treatment programmes.AIDS Res Ther. 2013 May 15;10(1):12. doi: 10.1186/1742-6405-10-12. AIDS Res Ther. 2013. PMID: 23675734 Free PMC article.
-
Operational research in Malawi: making a difference with cotrimoxazole preventive therapy in patients with tuberculosis and HIV.BMC Public Health. 2011 Jul 27;11:593. doi: 10.1186/1471-2458-11-593. BMC Public Health. 2011. PMID: 21794154 Free PMC article.
-
Cotrimoxazole prophylaxis and tuberculosis risk among people living with HIV.PLoS One. 2014 Jan 8;9(1):e83750. doi: 10.1371/journal.pone.0083750. eCollection 2014. PLoS One. 2014. PMID: 24421903 Free PMC article.
-
Incidence of malaria by cotrimoxazole use in HIV-infected Ugandan adults on antiretroviral therapy: a randomised, placebo-controlled study.AIDS. 2016 Feb 20;30(4):635-44. doi: 10.1097/QAD.0000000000000956. AIDS. 2016. PMID: 26558729 Free PMC article. Clinical Trial.
-
Application of causal inference methods in the analyses of randomised controlled trials: a systematic review.Trials. 2018 Jan 10;19(1):23. doi: 10.1186/s13063-017-2381-x. Trials. 2018. PMID: 29321046 Free PMC article.
References
-
- Bloland PB, Redd SC, Kazembe P, Tembenu R, Wirima JJ, Campbell CC. Co-trimoxazole for childhood febrile illness in malaria-endemic regions. Lancet. 1991;337:518–520. - PubMed
-
- Jones JL, Hanson DL, Dworkin MS. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. MMWR CDC Surveill Summ. 1999;48:1–22. - PubMed
-
- Carr A, Tindall B, Brew BJ. Low-dose trimethoprim-sulfamethoxazole prophylaxis for toxoplasmic encephalitis in patients with AIDS. Ann Intern Med. 1992;117:106–111. - PubMed
-
- Anglaret X, Chêne G, Attia A, the Cotrimo-CI study group Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Lancet. 1999;353:1463–1468. - PubMed
-
- Wiktor SZ, Sassan-Morokro M, Grant AD. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d'Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

