Lipid packing determines protein-membrane interactions: challenges for apolipoprotein A-I and high density lipoproteins
- PMID: 20347719
- PMCID: PMC2883020
- DOI: 10.1016/j.bbamem.2010.03.019
Lipid packing determines protein-membrane interactions: challenges for apolipoprotein A-I and high density lipoproteins
Abstract
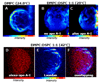
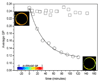
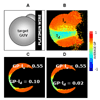
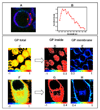
Protein and protein-lipid interactions, with and within specific areas in the cell membrane, are critical in order to modulate the cell signaling events required to maintain cell functions and viability. Biological bilayers are complex, dynamic platforms, and thus in vivo observations usually need to be preceded by studies on model systems that simplify and discriminate the different factors involved in lipid-protein interactions. Fluorescence microscopy studies using giant unilamellar vesicles (GUVs) as membrane model systems provide a unique methodology to quantify protein binding, interaction, and lipid solubilization in artificial bilayers. The large size of lipid domains obtainable on GUVs, together with fluorescence microscopy techniques, provides the possibility to localize and quantify molecular interactions. Fluorescence Correlation Spectroscopy (FCS) can be performed using the GUV model to extract information on mobility and concentration. Two-photon Laurdan Generalized Polarization (GP) reports on local changes in membrane water content (related to membrane fluidity) due to protein binding or lipid removal from a given lipid domain. In this review, we summarize the experimental microscopy methods used to study the interaction of human apolipoprotein A-I (apoA-I) in lipid-free and lipid-bound conformations with bilayers and natural membranes. Results described here help us to understand cholesterol homeostasis and offer a methodological design suited to different biological systems.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures