Diabetes induces changes in ILK, PINCH and components of related pathways in the spinal cord of rats
- PMID: 20347724
- PMCID: PMC2866122
- DOI: 10.1016/j.brainres.2010.03.067
Diabetes induces changes in ILK, PINCH and components of related pathways in the spinal cord of rats
Abstract
Recent work suggests that diabetes affects processing of peripheral, spinal and supraspinal signals in the spinal cord. However, there is little evidence for spinal cord lesions that would account for alterations in behavioral responses induced by experimental diabetes. Therefore, we assessed the expression of proteins that might affect neuronal cytoskeletal stability and thus promote dendritic and synaptic reorganization in diabetic rats. Expression of ILK, PINCH, PI3K, GSK-3beta, tau, MAP2, synaptophysin and drebrin in the lumbar spinal cord of non-diabetic and streptozotocin-diabetic rats was assessed by Western-blot analysis and immunocytochemistry after 8 and 20weeks of diabetes. The impact of diabetes on the proteins studied was duration-dependent with changes observed after 20 but not 8weeks of diabetes. ILK and PINCH proteins levels were significantly decreased and both colocalized to neurons and oligodendrocytes. PI3K protein levels were also significantly decreased, while GSK-3beta activity tended to be increased. Phosphorylation of tau and MAP2A/B protein expression were significantly increased, and expression of synaptophysin and drebrin were reduced in diabetic rats. Decreased ILK and PINCH as well as alterations of components of related signaling pathways are associated with tau hyperphosphorylation, MAP2 overexpression and reduction of synaptic proteins in the spinal cord of diabetic rats, suggesting that ILK and PINCH contribute to stabilization of axonal and dendritic structures. However, these changes are not likely the cause of altered behavioral responses in diabetic rats that occur after short-term diabetes, but may contribute to structural changes occurring in long-term diabetes.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures



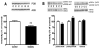


Similar articles
-
[Role of integrin-linked kinase signaling pathway in skin lesions and wound healing in diabetic rats].Zhonghua Shao Shang Za Zhi. 2016 Apr;32(4):216-23. doi: 10.3760/cma.j.issn.1009-2587.2016.04.006. Zhonghua Shao Shang Za Zhi. 2016. PMID: 27093933 Chinese.
-
Up-regulation of integrin-linked kinase in the streptozotocin-induced diabetic rat retina.Graefes Arch Clin Exp Ophthalmol. 2007 Oct;245(10):1523-32. doi: 10.1007/s00417-007-0616-3. Epub 2007 Jul 26. Graefes Arch Clin Exp Ophthalmol. 2007. PMID: 17653754
-
TSP-1 expression changes in diabetic rats with spinal cord injury.Neurol Res. 2009 Oct;31(8):878-82. doi: 10.1179/174313209X403887. Epub 2009 Mar 11. Neurol Res. 2009. PMID: 19278574
-
Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction.J Cell Sci. 1999 Dec;112 ( Pt 24):4485-9. doi: 10.1242/jcs.112.24.4485. J Cell Sci. 1999. PMID: 10574698
-
Posttranslational modifications of nerve cytoskeletal proteins in experimental diabetes.Mol Neurobiol. 1992 Summer-Fall;6(2-3):225-37. doi: 10.1007/BF02780555. Mol Neurobiol. 1992. PMID: 1476675
Cited by
-
Insulin attenuates apoptosis in neuronal cells by an integrin-linked kinase-dependent mechanism.Heliyon. 2019 Aug 16;5(8):e02294. doi: 10.1016/j.heliyon.2019.e02294. eCollection 2019 Aug. Heliyon. 2019. PMID: 31463398 Free PMC article.
-
Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine.Front Immunol. 2018 Mar 12;9:494. doi: 10.3389/fimmu.2018.00494. eCollection 2018. Front Immunol. 2018. PMID: 29593735 Free PMC article.
-
Synaptic input changes to spinal cord motoneurons correlate with motor control impairments in a type 1 diabetes mellitus model.Brain Behav. 2015 Sep 9;5(10):e00372. doi: 10.1002/brb3.372. eCollection 2015 Oct. Brain Behav. 2015. PMID: 26516607 Free PMC article.
-
Neuronal PINCH is regulated by TNF-α and is required for neurite extension.J Neuroimmune Pharmacol. 2011 Sep;6(3):330-40. doi: 10.1007/s11481-010-9236-5. Epub 2010 Aug 6. J Neuroimmune Pharmacol. 2011. PMID: 20689998 Free PMC article.
-
Unraveling Alzheimer's: Making Sense of the Relationship between Diabetes and Alzheimer's Disease1.J Alzheimers Dis. 2016;51(4):961-77. doi: 10.3233/JAD-150980. J Alzheimers Dis. 2016. PMID: 26967215 Free PMC article. Review.
References
-
- Ahroni JH, Boyko EJ, Davignon DR, Pecoraro RE. The health and functional status of veterans with diabetes. Diabetes Care. 1994;17:318–21. - PubMed
-
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A. 1997;94:298–303. - PMC - PubMed
-
- Arendt T. Disturbance of neuronal plasticity is a critical pathogenetic event in Alzheimer's disease. Int J Dev Neurosci. 2001;19:231–45. - PubMed
-
- Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

