The pros and cons of phytoestrogens
- PMID: 20347861
- PMCID: PMC3074428
- DOI: 10.1016/j.yfrne.2010.03.003
The pros and cons of phytoestrogens
Abstract
Phytoestrogens are plant derived compounds found in a wide variety of foods, most notably soy. A litany of health benefits including a lowered risk of osteoporosis, heart disease, breast cancer, and menopausal symptoms, are frequently attributed to phytoestrogens but many are also considered endocrine disruptors, indicating that they have the potential to cause adverse health effects as well. Consequently, the question of whether or not phytoestrogens are beneficial or harmful to human health remains unresolved. The answer is likely complex and may depend on age, health status, and even the presence or absence of specific gut microflora. Clarity on this issue is needed because global consumption is rapidly increasing. Phytoestrogens are present in numerous dietary supplements and widely marketed as a natural alternative to estrogen replacement therapy. Soy infant formula now constitutes up to a third of the US market, and soy protein is now added to many processed foods. As weak estrogen agonists/antagonists with molecular and cellular properties similar to synthetic endocrine disruptors such as Bisphenol A (BPA), the phytoestrogens provide a useful model to comprehensively investigate the biological impact of endocrine disruptors in general. This review weighs the evidence for and against the purported health benefits and adverse effects of phytoestrogens.
Copyright © 2010. Published by Elsevier Inc.
Figures


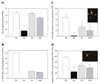
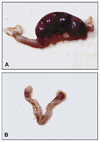
Similar articles
-
Endocrine disruption by dietary phyto-oestrogens: impact on dimorphic sexual systems and behaviours.Proc Nutr Soc. 2017 May;76(2):130-144. doi: 10.1017/S0029665116000677. Epub 2016 Jul 8. Proc Nutr Soc. 2017. PMID: 27389644 Free PMC article. Review.
-
Metabolism and health effects of phyto-estrogens.Crit Rev Food Sci Nutr. 2017 Jul 24;57(11):2432-2454. doi: 10.1080/10408398.2015.1077194. Crit Rev Food Sci Nutr. 2017. PMID: 26558495
-
Soy as an endocrine disruptor: cause for caution?J Pediatr Endocrinol Metab. 2010 Sep;23(9):855-61. doi: 10.1515/jpem.2010.138. J Pediatr Endocrinol Metab. 2010. PMID: 21175082 Review.
-
[Endocrine disruptor compounds and their role in the developmental programming of the reproductive axis].Rev Invest Clin. 2007 Jan-Feb;59(1):73-81. Rev Invest Clin. 2007. PMID: 17569302 Review. Spanish.
-
Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis.Drug Saf. 2001;24(9):665-82. doi: 10.2165/00002018-200124090-00003. Drug Saf. 2001. PMID: 11522120 Review.
Cited by
-
Transcriptional profiling of Chinese medicinal formula Si-Wu-Tang on breast cancer cells reveals phytoestrogenic activity.BMC Complement Altern Med. 2013 Jan 10;13:11. doi: 10.1186/1472-6882-13-11. BMC Complement Altern Med. 2013. PMID: 23305139 Free PMC article.
-
The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms.Nutrients. 2016 Sep 21;8(9):581. doi: 10.3390/nu8090581. Nutrients. 2016. PMID: 27657126 Free PMC article. Review.
-
Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review.Nutrients. 2022 Aug 26;14(17):3519. doi: 10.3390/nu14173519. Nutrients. 2022. PMID: 36079777 Free PMC article.
-
Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells.Int J Mol Med. 2020 Jun;45(6):1721-1734. doi: 10.3892/ijmm.2020.4556. Epub 2020 Mar 30. Int J Mol Med. 2020. PMID: 32236566 Free PMC article.
-
Evaluation of a bioprocessed soybean meal on nursery pig performance and immune status.J Anim Sci. 2017 Nov;95(11):5030-5039. doi: 10.2527/jas2017.1679. J Anim Sci. 2017. PMID: 29293734 Free PMC article.
References
-
- Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J. Anim. Sci. 1995;73:1509–1515. - PubMed
-
- Adams NR. Organizational and activational effects of phytoestrogens on the reproductive tract of the ewe. Proc. Soc. Exp. Biol. Med. 1995;208:87–91. - PubMed
-
- Adlercreutz H, Mazur W. Phyto-oestrogens and western diseases. Ann. Med. 1997;29:95–120. - PubMed
-
- Adlercreutz H, Mousavi Y, Clark J, Höckersted K, Hämäläinen EK, Wähälä K, Mäkelä T, Hase T. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J. Steroid Biochem. Mol. Biol. 1992;41:331–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

