Heat shock protein 70B' (HSP70B') expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages
- PMID: 20348092
- PMCID: PMC2871467
- DOI: 10.1074/jbc.M110.113605
Heat shock protein 70B' (HSP70B') expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages
Abstract
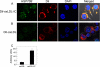
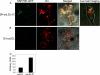
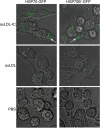
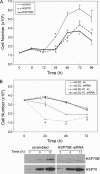
Heat shock proteins (HSPs) have been implicated in the activation and survival of macrophages. This study examined the role of HSP70B', a poorly characterized member of the HSP70 family, in response to oxidatively modified LDL (oxLDL) and immune complexes prepared with human oxLDL and purified human antibodies to oxLDL (oxLDL-IC) in monocytic and macrophage cell lines. Immunoblot analysis of cell lysates and conditioned medium from U937 cells treated with oxLDL alone revealed an increase in intracellular HSP70B' protein levels accompanied by a concomitant increase in HSP70B' extracellular levels. Fluorescence immunohistochemistry and confocal microscopy, however, demonstrated that oxLDL-IC stimulated the release of HSP70B', which co-localized with cell-associated oxLDL-IC. In HSP70B'-green fluorescent protein-transfected mouse RAW 264.7 cells, oxLDL-IC-induced HSP70B' co-localized with membrane-associated oxLDL-IC as well as the lipid moiety of internalized oxLDL-IC. Furthermore, the data demonstrated that HSP70B' is involved in cell survival, and this effect could be mediated by sphingosine kinase 1 (SK1) activation. An examination of regularly implicated cytokines revealed a significant relationship between HSP70B' and the release of the anti-inflammatory cytokine interleukin-10 (IL-10). Small interfering RNA knockdown of HSP70B' resulted in a corresponding decrease in SK1 mRNA levels and SK1 phosphorylation as well as increased release of IL-10. In conclusion, these findings suggest that oxLDL-IC induce the synthesis and release of HSP70B', and once stimulated, HSP70B' binds to the cell-associated and internalized lipid moiety of oxLDL-IC. The data also implicate HSP70B' in key cellular functions, such as regulation of SK1 activity and release of IL-10, which influence macrophage activation and survival.
Figures



Similar articles
-
Differential trafficking of oxidized LDL and oxidized LDL immune complexes in macrophages: impact on oxidative stress.PLoS One. 2010 Sep 2;5(9):e12534. doi: 10.1371/journal.pone.0012534. PLoS One. 2010. PMID: 20824093 Free PMC article.
-
Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release.Immunology. 2012 May;136(1):30-45. doi: 10.1111/j.1365-2567.2012.03552.x. Immunology. 2012. PMID: 22236141 Free PMC article.
-
Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells.Prostaglandins Other Lipid Mediat. 2006 Mar;79(1-2):126-40. doi: 10.1016/j.prostaglandins.2005.12.004. Epub 2006 Jan 31. Prostaglandins Other Lipid Mediat. 2006. PMID: 16516816
-
Clinical significance of the humoral immune response to modified LDL.Clin Immunol. 2010 Jan;134(1):55-65. doi: 10.1016/j.clim.2009.04.001. Epub 2009 May 8. Clin Immunol. 2010. PMID: 19427818 Free PMC article. Review.
-
Pathogenic role of modified LDL antibodies and immune complexes in atherosclerosis.J Atheroscler Thromb. 2013;20(10):743-54. doi: 10.5551/jat.19281. Epub 2013 Aug 20. J Atheroscler Thromb. 2013. PMID: 23965492 Review.
Cited by
-
Differential trafficking of oxidized LDL and oxidized LDL immune complexes in macrophages: impact on oxidative stress.PLoS One. 2010 Sep 2;5(9):e12534. doi: 10.1371/journal.pone.0012534. PLoS One. 2010. PMID: 20824093 Free PMC article.
-
Accelerated vascular disease in systemic lupus erythematosus: role of macrophage.Clin Immunol. 2015 Apr;157(2):133-44. doi: 10.1016/j.clim.2015.01.008. Epub 2015 Jan 28. Clin Immunol. 2015. PMID: 25638414 Free PMC article. Review.
-
Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release.Immunology. 2012 May;136(1):30-45. doi: 10.1111/j.1365-2567.2012.03552.x. Immunology. 2012. PMID: 22236141 Free PMC article.
-
Rescuing of deficient killing and phagocytic activities of macrophages derived from non-obese diabetic mice by treatment with geldanamycin or heat shock: potential clinical implications.Cell Stress Chaperones. 2011 Sep;16(5):573-81. doi: 10.1007/s12192-011-0268-4. Epub 2011 May 29. Cell Stress Chaperones. 2011. PMID: 21626279 Free PMC article.
-
Pharmacological Modulation of the Unfolded Protein Response as a Therapeutic Approach in Cutaneous T-Cell Lymphoma.Biomolecules. 2025 Jan 7;15(1):76. doi: 10.3390/biom15010076. Biomolecules. 2025. PMID: 39858470 Free PMC article.
References
-
- Morimoto R. I. (1993) Science 259, 1409–1410 - PubMed
-
- Xu Q., Wick G. (1996) Mol. Med. Today 2, 372–379 - PubMed
-
- Pockley A. G. (2002) Circulation 105, 1012–1017 - PubMed
-
- Roma P., Catapano A. L. (1996) Atherosclerosis 127, 147–154 - PubMed
-
- Johnson A. D., Berberian P. A., Tytell M., Bond M. G. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 27–36 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

