Expression of the anti-amyloidogenic secretase ADAM10 is suppressed by its 5'-untranslated region
- PMID: 20348102
- PMCID: PMC2871442
- DOI: 10.1074/jbc.M110.110742
Expression of the anti-amyloidogenic secretase ADAM10 is suppressed by its 5'-untranslated region
Abstract
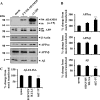
Proteolytic processing of the amyloid precursor protein by alpha-secretase prevents formation of the amyloid beta-peptide (Abeta), which is the main constituent of amyloid plaques in brains of Alzheimer disease (AD) patients. alpha-Secretase activity is decreased in AD, and overexpression of the alpha-secretase ADAM10 (a disintegrin and metalloprotease 10) in an AD animal model prevents amyloid pathology. ADAM10 has a 444-nucleotide-long, very GC-rich 5'-untranslated region (5'-UTR) with two upstream open reading frames. Because similar properties of 5'-UTRs are found in transcripts of many genes, which are regulated by translational control mechanisms, we asked whether ADAM10 expression is translationally controlled by its 5'-UTR. We demonstrate that the 5'-UTR of ADAM10 represses the rate of ADAM10 translation. In the absence of the 5'-UTR, we observed a significant increase of ADAM10 protein levels in HEK293 cells, whereas mRNA levels were not changed. Moreover, the 5'-UTR of ADAM10 inhibits translation of a luciferase reporter in an in vitro transcription/translation assay. Successive deletion of the first half of the ADAM10 5'-UTR revealed a striking increase in ADAM10 protein expression in HEK293 cells, suggesting that this part of the 5'-UTR contains inhibitory elements for translation. Moreover, we detect an enhanced alpha-secretase activity and consequently reduced Abeta levels in the conditioned medium of HEK293 cells expressing both amyloid precursor protein and a 5'-UTR-ADAM10 deletion construct lacking the first half of the 5'-UTR. Thus, we provide evidence that the 5'-UTR of ADAM10 may have an important role for post-transcriptional regulation of ADAM10 expression and consequently Abeta production.
Figures

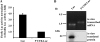
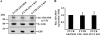


Similar articles
-
Regulation of α-secretase ADAM10 expression and activity.Exp Brain Res. 2012 Apr;217(3-4):343-52. doi: 10.1007/s00221-011-2885-7. Epub 2011 Oct 4. Exp Brain Res. 2012. PMID: 21969210 Review.
-
Translational repression of the disintegrin and metalloprotease ADAM10 by a stable G-quadruplex secondary structure in its 5'-untranslated region.J Biol Chem. 2011 Dec 30;286(52):45063-72. doi: 10.1074/jbc.M111.296921. Epub 2011 Nov 7. J Biol Chem. 2011. PMID: 22065584 Free PMC article.
-
MicroRNA-144 is regulated by activator protein-1 (AP-1) and decreases expression of Alzheimer disease-related a disintegrin and metalloprotease 10 (ADAM10).J Biol Chem. 2013 May 10;288(19):13748-61. doi: 10.1074/jbc.M112.381392. Epub 2013 Apr 1. J Biol Chem. 2013. PMID: 23546882 Free PMC article.
-
Discovery of Small Molecules for Up-Regulating the Translation of Antiamyloidogenic Secretase, a Disintegrin and Metalloproteinase 10 (ADAM10), by Binding to the G-Quadruplex-Forming Sequence in the 5' Untranslated Region (UTR) of Its mRNA.J Med Chem. 2015 May 14;58(9):3875-91. doi: 10.1021/acs.jmedchem.5b00139. Epub 2015 Apr 15. J Med Chem. 2015. PMID: 25822852
-
Upregulation of the alpha-secretase ADAM10--risk or reason for hope?FEBS J. 2010 Apr;277(7):1585-96. doi: 10.1111/j.1742-4658.2010.07566.x. Epub 2010 Feb 3. FEBS J. 2010. PMID: 20136654 Review.
Cited by
-
Regulation of Alpha-Secretase ADAM10 In vitro and In vivo: Genetic, Epigenetic, and Protein-Based Mechanisms.Front Mol Neurosci. 2017 Mar 17;10:56. doi: 10.3389/fnmol.2017.00056. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28367112 Free PMC article. Review.
-
Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE.Science. 2012 Jan 13;335(6065):225-8. doi: 10.1126/science.1214400. Science. 2012. PMID: 22246777 Free PMC article.
-
Dendritic Spines in Alzheimer's Disease: How the Actin Cytoskeleton Contributes to Synaptic Failure.Int J Mol Sci. 2020 Jan 30;21(3):908. doi: 10.3390/ijms21030908. Int J Mol Sci. 2020. PMID: 32019166 Free PMC article. Review.
-
Regulation of α-secretase ADAM10 expression and activity.Exp Brain Res. 2012 Apr;217(3-4):343-52. doi: 10.1007/s00221-011-2885-7. Epub 2011 Oct 4. Exp Brain Res. 2012. PMID: 21969210 Review.
-
ATP8B1 gene expression is driven by a housekeeping-like promoter independent of bile acids and farnesoid X receptor.PLoS One. 2012;7(12):e51650. doi: 10.1371/journal.pone.0051650. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251605 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

