Interaction between DNA Polymerase lambda and anticancer nucleoside analogs
- PMID: 20348107
- PMCID: PMC2878035
- DOI: 10.1074/jbc.M109.094391
Interaction between DNA Polymerase lambda and anticancer nucleoside analogs
Abstract
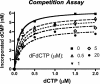
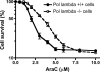
The anticancer activity of cytarabine (AraC) and gemcitabine (dFdC) is thought to result from chain termination after incorporation into DNA. To investigate their incorporation into DNA at atomic level resolution, we present crystal structures of human DNA polymerase lambda (Pol lambda) bound to gapped DNA and containing either AraC or dFdC paired opposite template dG. These structures reveal that AraC and dFdC can bind within the nascent base pair binding pocket of Pol lambda. Although the conformation of the ribose of AraCTP is similar to that of normal dCTP, the conformation of dFdCTP is significantly different. Consistent with these structures, Pol lambda efficiently incorporates AraCTP but not dFdCTP. The data are consistent with the possibility that Pol lambda could modulate the cytotoxic effect of AraC.
Figures

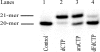

Similar articles
-
Effects of gemcitabine and araC on in vitro DNA synthesis mediated by the human breast cell DNA synthesome.Cancer Chemother Pharmacol. 2000;45(4):320-8. doi: 10.1007/s002800050047. Cancer Chemother Pharmacol. 2000. PMID: 10755321
-
Incorporation of gemcitabine and cytarabine into DNA by DNA polymerase beta and ligase III/XRCC1.Biochemistry. 2010 Jun 15;49(23):4833-40. doi: 10.1021/bi100200c. Biochemistry. 2010. PMID: 20459144
-
Polymerization of the triphosphates of AraC, 2',2'-difluorodeoxycytidine (dFdC) and OSI-7836 (T-araC) by human DNA polymerase alpha and DNA primase.Biochem Pharmacol. 2004 Dec 15;68(12):2337-46. doi: 10.1016/j.bcp.2004.07.042. Biochem Pharmacol. 2004. PMID: 15548380
-
Structural basis for topoisomerase I inhibition by nucleoside analogs.Nucleosides Nucleotides Nucleic Acids. 2003 May-Aug;22(5-8):653-8. doi: 10.1081/NCN-120022604. Nucleosides Nucleotides Nucleic Acids. 2003. PMID: 14565246 Review.
-
Cellular pharmacology of gemcitabine.Ann Oncol. 2006 May;17 Suppl 5:v7-12. doi: 10.1093/annonc/mdj941. Ann Oncol. 2006. PMID: 16807468 Review.
Cited by
-
Gemcitabine resistance in triple-negative breast cancer cells can be reverted by Drosophila melanogaster deoxyribonucleoside kinase in the nucleus or cytosol.Oncol Lett. 2020 Nov;20(5):247. doi: 10.3892/ol.2020.12109. Epub 2020 Sep 15. Oncol Lett. 2020. PMID: 32973960 Free PMC article.
-
Mitochondrial DNA damage and its consequences for mitochondrial gene expression.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):979-91. doi: 10.1016/j.bbagrm.2012.06.002. Epub 2012 Jun 19. Biochim Biophys Acta. 2012. PMID: 22728831 Free PMC article. Review.
-
DNA lesion bypass polymerases and 4'-thio-β-Darabinofuranosylcytosine (T-araC).Int J Biochem Mol Biol. 2011;2(4):340-6. Epub 2011 Nov 25. Int J Biochem Mol Biol. 2011. PMID: 22187668 Free PMC article.
-
Design of amino acid- and carbohydrate-based anticancer drugs to inhibit polymerase η.Sci Rep. 2022 Nov 2;12(1):18461. doi: 10.1038/s41598-022-22810-z. Sci Rep. 2022. PMID: 36323739 Free PMC article.
-
Getting Lost in the Cell-Lysosomal Entrapment of Chemotherapeutics.Cancers (Basel). 2020 Dec 7;12(12):3669. doi: 10.3390/cancers12123669. Cancers (Basel). 2020. PMID: 33297435 Free PMC article. Review.
References
-
- Balzarini J. (1994) Pharm. World Sci. 16, 113–126 - PubMed
-
- Hiddemann W. (1991) Ann. Hematol. 62, 119–128 - PubMed
-
- Burkes R. L., Shepherd F. A. (1995) Ann. Oncol. 6, Suppl. 3, S57–S60 - PubMed
-
- Possinger K. (1995) Anticancer Drugs 6, Suppl. 6, 55–59 - PubMed
-
- King R. S. (1996) Cancer Pract. 4, 353–354 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

