Antibiotics for the treatment of dysentery in children
- PMID: 20348130
- PMCID: PMC2845863
- DOI: 10.1093/ije/dyq024
Antibiotics for the treatment of dysentery in children
Abstract
Background: Ciprofloxacin, ceftriaxone and pivmecillinam are the antibiotics currently recommended by the World Health Organization (WHO) for the treatment of dysentery in children; yet there have been no reviews of the clinical effectiveness of these antibiotics in recent years.
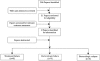
Methods: We reviewed all literature reporting the effect of ciprofloxacin, ceftriaxone and pivmecillinam for the treatment of dysentery in children in the developing countries. We used a standardized abstraction and grading format and performed meta-analyses to determine the effect of treatment with these antibiotics on rates of treatment failure, bacteriological failure and bacteriological relapse. The CHERG Standard Rules were applied to determine the final effect of treatment with these antibiotics on diarrhoea mortality.
Results: Eight papers were selected for abstraction. Treatment with ciprofloxacin, ceftriaxone or pivmecillinam resulted in a cure rate of >99% while assessing clinical failure, bacteriological failure and bacteriological relapse.
Conclusions: The antibiotics recommended by the WHO--ciprofloxacin, ceftriaxone and pivmecillinam--are effective in reducing the clinical and bacteriological signs and symptoms of dysentery and thus can be expected to decrease diarrhoea mortality attributable to dysentery.
Figures

References
-
- Guerin PJ, Brasher C, Baron E, et al. Case management of a multidrug-resistant Shigella dysenteriae serotype 1 outbreak in a crisis context in Sierra Leone, 1999–2000. Trans R Soc Trop Med Hyg. 2004;98:635–43. - PubMed
-
- Amieva MR. Important bacterial gastrointestinal pathogens in children: a pathogenesis perspective. Pediatr Clin North Am. 2005;52:749–77, vi. - PubMed
-
- Huppertz HI. An epidemic of bacillary dysentery in western Rwanda 1981–1982. Cent Afr J Med. 1986;32:79–82. - PubMed
-
- el Bushra HE, Bin Saeed AA. Intrafamilial person-to-person spread of bacillary dysentery due to Shigella dysenteriae in southwestern Saudi Arabia. East Afr Med J. 1999;76:255–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

