Development of a sarcoidosis murine lung granuloma model using Mycobacterium superoxide dismutase A peptide
- PMID: 20348207
- PMCID: PMC3049230
- DOI: 10.1165/rcmb.2009-0350OC
Development of a sarcoidosis murine lung granuloma model using Mycobacterium superoxide dismutase A peptide
Abstract
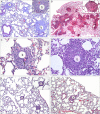
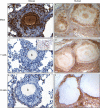
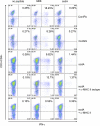
Sarcoidosis is characterized by noncaseating granulomas containing CD4(+) T cells with a Th1 immunophenotype. Although the causative antigens remain unknown, independent studies noted molecular and immunologic evidence of mycobacterial virulence factors in sarcoidosis specimens. A major limiting factor in discovering new insights into the pathogenesis of sarcoidosis is the lack of an animal model. Using a distinct superoxide dismutase A peptide (sodA) associated with sarcoidosis granulomas, we developed a pulmonary model of sarcoidosis granulomatous inflammation. Mice were sensitized by a subcutaneous injection of sodA, incorporated in incomplete Freund's adjuvant (IFA). Control subjects consisted of mice with no sensitization (ConNS), sensitized with IFA only (ConIFA), or with Schistosoma mansoni eggs. Fourteen days later, sensitized mice were challenged by tail-vein injection of naked beads, covalently coupled to sodA peptides or to schistosome egg antigens (SEA). Histologic analysis revealed hilar lymphadenopathy and noncaseating granulomas in the lungs of sodA-treated or SEA-treated mice. Flow cytometry of bronchoalveolar lavage (BAL) demonstrated CD4(+) T-cell responses against sodA peptide in the sodA-sensitized mice only. Cytometric bead analysis revealed significant differences in IL-2 and IFN-γ secretion in the BAL fluid of sodA-treated mice, compared with mice that received SEA or naked beads (P = 0.008, Wilcoxon rank sum test). ConNS and ConIFA mice demonstrated no significant formation of granuloma, and no Th1 immunophenotype. The use of microbial peptides distinct for sarcoidosis reveals a histologic and immunologic profile in the murine model that correlates well with those profiles noted in human sarcoidosis, providing the framework to investigate the molecular basis for the progression or resolution of sarcoidosis.
Figures




Similar articles
-
[Establishment and identification of a C57B/6 mouse sarcoidosis granuloma model].Zhonghua Jie He He Hu Xi Za Zhi. 2013 Aug;36(8):587-91. Zhonghua Jie He He Hu Xi Za Zhi. 2013. PMID: 24252735 Chinese.
-
Superoxide dismutase A antigens derived from molecular analysis of sarcoidosis granulomas elicit systemic Th-1 immune responses.Respir Res. 2008 Apr 25;9(1):36. doi: 10.1186/1465-9921-9-36. Respir Res. 2008. PMID: 18439270 Free PMC article.
-
Multiple Mycobacterium antigens induce interferon-gamma production from sarcoidosis peripheral blood mononuclear cells.Clin Exp Immunol. 2007 Dec;150(3):460-8. doi: 10.1111/j.1365-2249.2007.03510.x. Epub 2007 Oct 9. Clin Exp Immunol. 2007. PMID: 17924974 Free PMC article.
-
Vitamin D, innate immunity, and sarcoidosis granulomatous inflammation: insights from mycobacterial research.Curr Opin Pulm Med. 2010 Sep;16(5):461-4. doi: 10.1097/MCP.0b013e32833af7e8. Curr Opin Pulm Med. 2010. PMID: 20473167 Review.
-
Th17-lineage cells in pulmonary sarcoidosis and Löfgren's syndrome: Friend or foe?J Autoimmun. 2018 Feb;87:82-96. doi: 10.1016/j.jaut.2017.12.012. Epub 2018 Jan 5. J Autoimmun. 2018. PMID: 29310925 Review.
Cited by
-
Localization of propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium.Mod Pathol. 2012 Sep;25(9):1284-97. doi: 10.1038/modpathol.2012.80. Epub 2012 May 18. Mod Pathol. 2012. PMID: 22596102 Free PMC article.
-
Models Contribution to the Understanding of Sarcoidosis Pathogenesis: "Are There Good Models of Sarcoidosis?".J Clin Med. 2020 Jul 31;9(8):2445. doi: 10.3390/jcm9082445. J Clin Med. 2020. PMID: 32751786 Free PMC article. Review.
-
Induction of Pulmonary Granuloma Formation by Propionibacterium acnes Is Regulated by MyD88 and Nox2.Am J Respir Cell Mol Biol. 2017 Jan;56(1):121-130. doi: 10.1165/rcmb.2016-0035OC. Am J Respir Cell Mol Biol. 2017. PMID: 27607191 Free PMC article.
-
Evidence for mycobacteria in sarcoidosis.Am J Respir Cell Mol Biol. 2011 Nov;45(5):899-905. doi: 10.1165/rcmb.2010-0433TR. Epub 2011 Jun 9. Am J Respir Cell Mol Biol. 2011. PMID: 21659662 Free PMC article. Review.
-
Novel three-dimensional biochip pulmonary sarcoidosis model.PLoS One. 2021 Feb 4;16(2):e0245805. doi: 10.1371/journal.pone.0245805. eCollection 2021. PLoS One. 2021. PMID: 33539409 Free PMC article.
References
-
- Ichikawa H, Kataoka M, Hiramatsu J, Ohmori M, Tanimoto Y, Kanehiro A, Nakata Y, Tanimoto M. Quantitative analysis of propionibacterial dna in bronchoalveolar lavage cells from patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2008;25:15–20. - PubMed
-
- Dubaniewicz A, Dubaniewicz-Wybieralska M, Sternau A, Zwolska Z, Izycka-Swieszewska E, Augustynowicz-Kopec E, Skokowski J, Singh M, Zimnoch L. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J Clin Microbiol 2006;44:3448–3451. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

