Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model
- PMID: 20348235
- PMCID: PMC2861093
- DOI: 10.2353/ajpath.2010.091069
Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model
Abstract
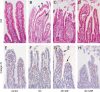
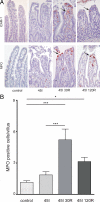
Human intestinal ischemia-reperfusion (IR) is a frequent phenomenon carrying high morbidity and mortality. Although intestinal IR-induced inflammation has been studied extensively in animal models, human intestinal IR induced inflammatory responses remain to be characterized. Using a newly developed human intestinal IR model, we show that human small intestinal ischemia results in massive leakage of intracellular components from ischemically damaged cells, as indicated by increased arteriovenous concentration differences of intestinal fatty acid binding protein and soluble cytokeratin 18. IR-induced intestinal barrier integrity loss resulted in free exposure of the gut basal membrane (collagen IV staining) to intraluminal contents, which was accompanied by increased arteriovenous concentration differences of endotoxin. Western blot for complement activation product C3c and immunohistochemistry for activated C3 revealed complement activation after IR. In addition, intestinal IR resulted in enhanced tissue mRNA expression of IL-6, IL-8, and TNF-alpha, which was accompanied by IL-6 and IL-8 release into the circulation. Expression of intercellular adhesion molecule-1 was markedly increased during reperfusion, facilitating influx of neutrophils into IR-damaged villus tips. In conclusion, this study for the first time shows the sequelae of human intestinal IR-induced inflammation, which is characterized by complement activation, production and release of cytokines into the circulation, endothelial activation, and neutrophil influx into IR-damaged tissue.
Figures


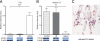
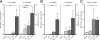
References
-
- American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology. 2000;118:951–953. - PubMed
-
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545–564. - PubMed
-
- Kubes P, Hunter J, Granger DN. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology. 1992;103:807–812. - PubMed
-
- Homer-Vanniasinkam S, Crinnion JN, Gough MJ. Post-ischaemic organ dysfunction: a review. Eur J Vasc Endovasc Surg. 1997;14:195–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

