Desmosomal plakophilins in the prostate and prostatic adenocarcinomas: implications for diagnosis and tumor progression
- PMID: 20348237
- PMCID: PMC2861115
- DOI: 10.2353/ajpath.2010.090737
Desmosomal plakophilins in the prostate and prostatic adenocarcinomas: implications for diagnosis and tumor progression
Abstract
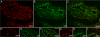
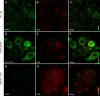
The plakophilins, members of the armadillo-repeat family, consist of three different proteins (PKP1-3) that are specifically recruited to desmosomal plaques in a highly cell type-specific manner. Using immunofluorescence, immunoelectron microscopy, and immunoblot, we found that all three plakophilins occurred in luminal and basal cells of the pseudostratified prostate epithelium. The analysis of 135 cases of prostatic adenocarcinomas grouped into tumors with low (Gleason score < or = 6), intermediate (Gleason score 7), and high Gleason score (8 < or = Gleason score < or = 10) showed that the expression of PKP1 was reduced or lost in adenocarcinomas with high Gleason scores. The expression of PKP2 was unchanged in all prostatic adenocarcinomas analyzed. In contrast, PKP3 expression was increased in carcinomas with high Gleason scores in comparison with carcinomas with low Gleason scores. In DU 145 cell lines with either overexpression or knockdown of PKP3, both imbalances resulted in fewer desmosomal cell contacts. In addition, overexpression of PKP3 in DU 145 cells led to an augmentation in proliferation rate. Our data imply that both loss of PKP1 and up-regulation of PKP3 expression are biologically important events in prostate cancer and are associated with a more aggressive phenotype.
Figures







Similar articles
-
Plakophilin-associated RNA-binding proteins in prostate cancer and their implications in tumor progression and metastasis.Virchows Arch. 2013 Sep;463(3):379-90. doi: 10.1007/s00428-013-1452-y. Epub 2013 Jul 24. Virchows Arch. 2013. PMID: 23881279
-
Expression of plakophilins (PKP1, PKP2, and PKP3) in gastric cancers.Diagn Pathol. 2011 Jan 2;6:1. doi: 10.1186/1746-1596-6-1. Diagn Pathol. 2011. PMID: 21194493 Free PMC article.
-
c-Src mediated tyrosine phosphorylation of plakophilin 3 as a new mechanism to control desmosome composition in cells exposed to oxidative stress.Cell Tissue Res. 2015 Mar;359(3):799-816. doi: 10.1007/s00441-014-2063-x. Epub 2014 Dec 12. Cell Tissue Res. 2015. PMID: 25501895
-
Plakophilins in desmosomal adhesion and signaling.Cell Commun Adhes. 2014 Feb;21(1):25-42. doi: 10.3109/15419061.2013.876017. Cell Commun Adhes. 2014. PMID: 24460199 Review.
-
Role and function of plakophilin 3 in cancer progression and skin disease.Cancer Sci. 2024 Jan;115(1):17-23. doi: 10.1111/cas.16019. Epub 2023 Dec 4. Cancer Sci. 2024. PMID: 38048779 Free PMC article. Review.
Cited by
-
Holding Tight: Cell Junctions and Cancer Spread.Trends Cancer Res. 2012;8:61-69. Trends Cancer Res. 2012. PMID: 23450077 Free PMC article.
-
Desmosome regulation and signaling in disease.Cell Tissue Res. 2015 Jun;360(3):501-12. doi: 10.1007/s00441-015-2136-5. Epub 2015 Feb 19. Cell Tissue Res. 2015. PMID: 25693896 Free PMC article. Review.
-
Comprehensive Analysis Identifies PKP3 Overexpression in Pancreatic Cancer Related to Unfavorable Prognosis.Biomedicines. 2023 Sep 6;11(9):2472. doi: 10.3390/biomedicines11092472. Biomedicines. 2023. PMID: 37760912 Free PMC article.
-
Plakophilin-3 catenin associates with the ETV1/ER81 transcription factor to positively modulate gene activity.PLoS One. 2014 Jan 27;9(1):e86784. doi: 10.1371/journal.pone.0086784. eCollection 2014. PLoS One. 2014. PMID: 24475179 Free PMC article.
-
Mice with Hepatic Loss of the Desmosomal Protein γ-Catenin Are Prone to Cholestatic Injury and Chemical Carcinogenesis.Am J Pathol. 2015 Dec;185(12):3274-89. doi: 10.1016/j.ajpath.2015.08.019. Epub 2015 Oct 17. Am J Pathol. 2015. PMID: 26485505 Free PMC article.
References
-
- Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. - PubMed
-
- Hatzfeld M. The p120 family of cell adhesion molecules. Eur Cell Biol. 2005;84:205–214. - PubMed
-
- Schmidt A, Jäger S. Plakophilins—hard work in the desmosome, recreation in the nucleus? Eur J Cell Biol. 2005;84:189–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

