Pathological features of heterologous immunity are regulated by the private specificities of the immune repertoire
- PMID: 20348239
- PMCID: PMC2861077
- DOI: 10.2353/ajpath.2010.090656
Pathological features of heterologous immunity are regulated by the private specificities of the immune repertoire
Abstract
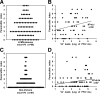
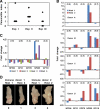
Heterologous immunity associated with cross-reactive T-cell responses is proposed to contribute to variations among individuals in the pathogenesis of human viral infections. In genetically identical mice with similar infection histories, marked variations in the magnitude and specificities of T-cell responses under conditions of heterologous immunity occur and have been linked to the private specificity of T-cell repertoires in individual immune mice. Variations in immunopathology in the form of panniculitis are observed in lymphocytic choriomeningitis virus-immune mice after vaccinia virus infection. By adoptively transferring splenocytes from individual lymphocytic choriomeningitis virus-immune donors into paired recipients, we show here that, on vaccinia virus infection, similar levels of panniculitis were generated in recipients from a single donor, but the severity of panniculitis varied among recipients receiving cells from different donors. This indicates that virus-induced immunopathology under conditions of heterologous immunity is a function of the private specificity of the immune repertoire.
Figures
Similar articles
-
Private specificities of CD8 T cell responses control patterns of heterologous immunity.J Exp Med. 2005 Feb 21;201(4):523-33. doi: 10.1084/jem.20041337. Epub 2005 Feb 14. J Exp Med. 2005. PMID: 15710651 Free PMC article.
-
Heterologous Immunity and Persistent Murine Cytomegalovirus Infection.J Virol. 2017 Jan 3;91(2):e01386-16. doi: 10.1128/JVI.01386-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807227 Free PMC article.
-
Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity.J Clin Invest. 2006 May;116(5):1443-56. doi: 10.1172/JCI27804. Epub 2006 Apr 13. J Clin Invest. 2006. PMID: 16614754 Free PMC article.
-
Evolution of the CD8 T-cell repertoire during infections.Microbes Infect. 2000 Jul;2(9):1025-39. doi: 10.1016/s1286-4579(00)01257-0. Microbes Infect. 2000. PMID: 10967283 Review.
-
The privacy of T cell memory to viruses.Curr Top Microbiol Immunol. 2006;311:117-53. doi: 10.1007/3-540-32636-7_5. Curr Top Microbiol Immunol. 2006. PMID: 17048707 Free PMC article. Review.
Cited by
-
Vaccination and heterologous immunity: educating the immune system.Trans R Soc Trop Med Hyg. 2015 Jan;109(1):62-9. doi: 10.1093/trstmh/tru198. Trans R Soc Trop Med Hyg. 2015. PMID: 25573110 Free PMC article. Review.
-
Antigen-Specificity of T Cell Infiltrates in Biopsies With T Cell-Mediated Rejection and BK Polyomavirus Viremia: Analysis by Next Generation Sequencing.Am J Transplant. 2016 Nov;16(11):3131-3138. doi: 10.1111/ajt.13911. Epub 2016 Jul 15. Am J Transplant. 2016. PMID: 27273900 Free PMC article.
-
Cross-reactivity influences changes in human influenza A virus and Epstein Barr virus specific CD8 memory T cell receptor alpha and beta repertoires between young and old.Front Immunol. 2023 Feb 24;13:1011935. doi: 10.3389/fimmu.2022.1011935. eCollection 2022. Front Immunol. 2023. PMID: 36923729 Free PMC article.
-
Large-Scale Structure-Based Screening of Potential T Cell Cross-Reactivities Involving Peptide-Targets From BCG Vaccine and SARS-CoV-2.Front Immunol. 2022 Jan 13;12:812176. doi: 10.3389/fimmu.2021.812176. eCollection 2021. Front Immunol. 2022. PMID: 35095907 Free PMC article.
-
The two-faced T cell epitope: examining the host-microbe interface with JanusMatrix.Hum Vaccin Immunother. 2013 Jul;9(7):1577-86. doi: 10.4161/hv.24615. Epub 2013 Apr 12. Hum Vaccin Immunother. 2013. PMID: 23584251 Free PMC article.
References
-
- Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–634. - PubMed
-
- Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

