Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells
- PMID: 20348242
- PMCID: PMC2861113
- DOI: 10.2353/ajpath.2010.090777
Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells
Abstract
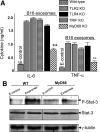
In this study we observed that mice pretreated with tumor exosomes had a significant acceleration of tumor metastasis in the lung. Tumor metastasis correlated significantly with an increase in recruitment of more Myeloid-derived suppressor cells (MDSCs) in the lung of C57BL/6j (B6) mice pretreated with tumor exosomes. These effects were blunted when MyD88 knockout (KO) mice were pretreated with tumor exosomes. MDSCs induced by tumor exosomes and isolated from wild-type B6 mice also more potently inhibited T cell activation and induction of interleukin-6 and tumor necrosis factor-alpha than MDSCs isolated from the lung of MyD88 KO mice. In vitro, addition of tumor exosomes to bone marrow-derived CD11b(+)Gr-1(+) cells isolated from wild-type B6 mice resulted in more cytokine production, including tumor necrosis factor-alpha, interleukin-6, and the chemokine CCL2, than CD11b(+)Gr-1(+) cells isolated from MyD88 KO mice. Moreover, lower levels of CCL2 were observed in the lungs in MyD88 KO mice pretreated with tumor exosomes than that in wild-type mice. Together these data demonstrate a pivotal role for MyD88 in tumor exosome-mediated expansion of MDSCs and tumor metastasis.
Figures

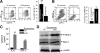



References
-
- Lee JJ, Lotze MT. Molecular basis of metastasis. N Engl J Med. 2009;360:1679. author reply 1679–1680. - PubMed
-
- Melnikova VO, Bar-Eli M. Inflammation and melanoma metastasis. Pigment Cell Melanoma Res. 2009;22:257–267. - PubMed
-
- Mon NN, Kokuryo T, Hamaguchi M. Inflammation and tumor progression: a lesson from TNF-alpha-dependent FAK signaling in cholangiocarcinoma. Methods Mol Biol. 2009;512:279–293. - PubMed
-
- Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang HG. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

