Fluconazole transport into Candida albicans secretory vesicles by the membrane proteins Cdr1p, Cdr2p, and Mdr1p
- PMID: 20348384
- PMCID: PMC2901649
- DOI: 10.1128/EC.00355-09
Fluconazole transport into Candida albicans secretory vesicles by the membrane proteins Cdr1p, Cdr2p, and Mdr1p
Abstract
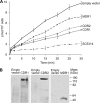
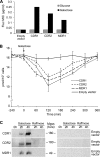
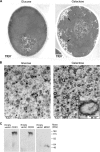
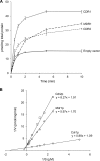
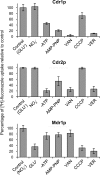
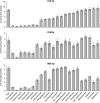
A major cause of azole resistance in Candida albicans is overexpression of CDR1, CDR2, and/or MDR1, which encode plasma membrane efflux pumps. To analyze the catalytic properties of these pumps, we used ACT1- and GAL1-regulated expression plasmids to overexpress CDR1, CDR2, or MDR1 in a C. albicans cdr1 cdr2 mdr1-null mutant. When the genes of interest were expressed, the resulting transformants were more resistant to multiple azole antifungals, and accumulated less [(3)H]fluconazole intracellularly, than empty-vector controls. Next, we used a GAL1-regulated dominant negative sec4 allele to cause cytoplasmic accumulation of post-Golgi secretory vesicles (PGVs), and we found that PGVs isolated from CDR1-, CDR2-, or MDR1-overexpressing cells accumulated much more [(3)H]fluconazole than did PGVs from empty-vector controls. The K(m)s (expressed in micromolar concentrations) and V(max)s (expressed in picomoles per milligram of protein per minute), respectively, for [(3)H]fluconazole transport were 0.8 and 0.91 for Cdr1p, 4.3 and 0.52 for Cdr2p, and 3.5 and 0.59 for Mdr1p. [(3)H]fluconazole transport by Cdr1p and Cdr2p required ATP and was unaffected by carbonyl cyanide 3-chlorophenylhydrazone (CCCP), whereas [(3)H]fluconazole transport by Mdr1p did not require ATP and was inhibited by CCCP. [(3)H]fluconazole uptake by all 3 pumps was inhibited by all other azoles tested, with 50% inhibitory concentrations (IC(50)s; expressed as proportions of the [(3)H]fluconazole concentration) of 0.2 to 5.6 for Cdr1p, 0.3 to 3.1 for Cdr2p, and 0.3 to 3.1 for Mdr1p. The methods used in this study may also be useful for studying other plasma membrane transporters in C. albicans and other medically important fungi.
Figures
References
-
- Banerjee D., Martin N., Nandi S., Shukla S., Dominguez A., Mukhopadhyay G., Prasad R. 2007. A genome-wide steroid response study of the major human fungal pathogen Candida albicans. Mycopathologia 164:1–17 - PubMed
-
- Cannon R. D., Fischer F. J., Niimi K., Niimi M., Arisawa M. 1998. Drug pumping mechanisms in Candida albicans. Nippon Ishinkin Gakkai Zasshi 39:73–78 - PubMed
-
- Chen C. G., Yang Y. L., Tseng K. Y., Shih H. I., Liou C. H., Lin C. C., Lo H. J. 2009. Rep1p negatively regulating MDR1 efflux pump involved in drug resistance in Candida albicans. Fungal Genet. Biol. 46:714–720 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

