Structural resolution of a tandem hormone-binding element in the insulin receptor and its implications for design of peptide agonists
- PMID: 20348418
- PMCID: PMC2872410
- DOI: 10.1073/pnas.1001813107
Structural resolution of a tandem hormone-binding element in the insulin receptor and its implications for design of peptide agonists
Abstract
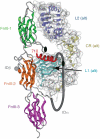
The C-terminal segment of the human insulin receptor alpha-chain (designated alphaCT) is critical to insulin binding as has been previously demonstrated by alanine scanning mutagenesis and photo-cross-linking. To date no information regarding the structure of this segment within the receptor has been available. We employ here the technique of thermal-factor sharpening to enhance the interpretability of the electron-density maps associated with the earlier crystal structure of the human insulin receptor ectodomain. The alphaCT segment is now resolved as being engaged with the central beta-sheet of the first leucine-rich repeat (L1) domain of the receptor. The segment is alpha-helical in conformation and extends 11 residues N-terminal of the classical alphaCT segment boundary originally defined by peptide mapping. This tandem structural element (alphaCT-L1) thus defines the intact primary insulin-binding surface of the apo-receptor. The structure, together with isothermal titration calorimetry data of mutant alphaCT peptides binding to an insulin minireceptor, leads to the conclusion that putative "insulin-mimetic" peptides in the literature act at least in part as mimics of the alphaCT segment as well as of insulin. Photo-cross-linking by novel bifunctional insulin derivatives demonstrates that the interaction of insulin with the alphaCT segment and the L1 domain occurs in trans, i.e., these components of the primary binding site are contributed by alternate alpha-chains within the insulin receptor homodimer. The tandem structural element defines a new target for the design of insulin agonists for the treatment of diabetes mellitus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
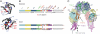
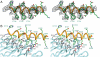

References
-
- De Meyts P. Insulin and its receptor: Structure, function and evolution. Bioessays. 2004;26:1351–1362. - PubMed
-
- Adams MJ, et al. Structure of rhombohedral 2 zinc insulin crystals. Nature. 1969;224:491–495.
-
- McKern NM, et al. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature. 2006;443:218–221. - PubMed
-
- Lawrence MC, McKern NM, Ward CW. Insulin receptor structure and its implications for the IGF-1 receptor. Curr Opin Struct Biol. 2007;17:699–705. - PubMed
-
- Ward CW, Lawrence MC, Streltsov VA, Adams TE, McKern NM. The insulin and EGF receptor structures: New insights into ligand-induced receptor activation. Trends Biochem Sci. 2007;32:129–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

