A comparison of two short-course primaquine regimens for the treatment and radical cure of Plasmodium vivax malaria in Thailand
- PMID: 20348496
- PMCID: PMC2844579
- DOI: 10.4269/ajtmh.2010.09-0428
A comparison of two short-course primaquine regimens for the treatment and radical cure of Plasmodium vivax malaria in Thailand
Abstract
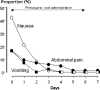
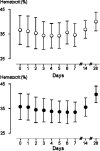
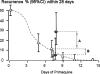
Thai adult males (N = 85) with acute Plasmodium vivax malaria and normal glucose-6-phosphate dehydrogenase screening were randomized to receive 30 mg or 60 mg primaquine daily for 7 days (N = 43 and 42, respectively). The regimens were well tolerated and all patients recovered fully. Median fever clearance (47 hours; range 4 to 130 hours), mean + or - SD parasite clearance times (87.7 + or - 25.3 hours), gametocyte clearance, and adverse effects were similar in the 2 groups. Two patients, 1 from each group, had a 30% reduction in hematocrit. The cumulative 28 day relapse rate (95% confidence interval) by Kaplan Meier survival analysis was 29% (16-49%) in the 30 mg group compared with 7% (2-24%) in the 60 mg group; P = 0.027. Comparison with previous data obtained at this same site suggests that the recurrences comprised approximately 17% recrudescences and 12% relapses in the 30 mg/day group compared with 3% recrudescences and 4% relapses in the 60 mg/day group. These data suggest that the dose-response relationships for primaquine's asexual stage and hypnozoitocidal activities in-vivo are different. A 1 week course of primaquine 60 mg daily is an effective treatment of vivax malaria in this region.
Figures

References
-
- Pukrittayakamee S, Vanijanonta S, Chantra A, Clemens R, White NJ. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J Infect Dis. 1994;169:932–935. - PubMed
-
- Baird JK, Lacy MD, Basri H, Barcus MJ, Maguire JD, Bangs MJ, Gramzinski R, Sismadi P, Krisin, Ling J, Wiady I, Kusumaningsih M, Jones TR, Fryauff DJ, Hoffman SL. Randomized, parallel placebo-controlled trial of primaquine for malaria prophylaxis in Papua, Indonesia. Clin Infect Dis. 2001;33:1990–1997. - PubMed
-
- Soto J, Toledo J, Rodriquez M, Sanchez J, Herrera R, Padilla J, Berman J. Primaquine prophylaxis against malaria in nonimmune Colombian soldiers: efficacy and toxicity. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129:241–244. - PubMed
-
- Bunnag D, Karbwang J, Thanavibul A, Chittamas S, Ratanapongse Y, Chalermrut K, Bangchang KN, Harinasuta T. High dose of primaquine in primaquine resistant vivax malaria. Trans R Soc Trop Med Hyg. 1994;88:218–219. - PubMed
-
- Wilairatana P, Silachamroon U, Krudsood S, Singhasivanon P, Treeprasertsuk S, Bussaratid V, Phumratanaprapin W, Srivilirit S, Looareesuwan S. Efficacy of primaquine regimens for primaquine-resistant Plasmodium vivax malaria in Thailand. Am J Trop Med Hyg. 1999;61:973–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

