Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)alpha1-specific activity and glucose transport in human skeletal muscle
- PMID: 20349036
- PMCID: PMC2860569
- DOI: 10.1007/s00125-010-1716-x
Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)alpha1-specific activity and glucose transport in human skeletal muscle
Abstract
Aims/hypothesis: We investigated the direct effect of a nitric oxide donor (spermine NONOate) on glucose transport in isolated human skeletal muscle and L6 skeletal muscle cells. We hypothesised that pharmacological treatment of human skeletal muscle with N-(2-aminoethyl)-N-(2-hydroxy-2-nitrosohydrazino)-1,2-ethylenediamine (spermine NONOate) would increase intracellular cyclic GMP (cGMP) levels and promote glucose transport.
Methods: Skeletal muscle strips were prepared from vastus lateralis muscle biopsies obtained from seven healthy men. Muscle strips were incubated in the absence or presence of 5 mmol/l spermine NONOate or 120 nmol/l insulin. The L6 muscle cells were treated with spermine NONOate (20 micromol/l) and incubated in the absence or presence of insulin (120 nmol/l). The direct effect of spermine NONOate and insulin on glucose transport, cGMP levels and signal transduction was determined.
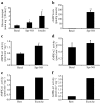
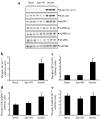
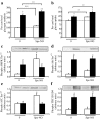
Results: In human skeletal muscle, spermine NONOate increased glucose transport 2.4-fold (p < 0.05), concomitant with increased cGMP levels (80-fold, p < 0.001). Phosphorylation of components of the canonical insulin signalling cascade was unaltered by spermine NONOate exposure, implicating an insulin-independent signalling mechanism. Consistent with this, spermine NONOate increased AMP-activated protein kinase (AMPK)-alpha1-associated activity (1.7-fold, p < 0.05). In L6 muscle cells, spermine NONOate increased glucose uptake (p < 0.01) and glycogen synthesis (p < 0.001), an effect that was in addition to that of insulin. Spermine NONOate also elicited a concomitant increase in AMPK and acetyl-CoA carboxylase phosphorylation. In the presence of the guanylate cyclase inhibitor LY-83583 (10 micromol/l), spermine NONOate had no effect on glycogen synthesis and AMPK-alpha1 phosphorylation.
Conclusions/interpretation: Pharmacological treatment of skeletal muscle with spermine NONOate increases glucose transport via insulin-independent signalling pathways involving increased intracellular cGMP levels and AMPK-alpha1-associated activity.
Figures
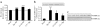
Similar articles
-
L-Arginine enhances glucose and lipid metabolism in rat L6 myotubes via the NO/ c-GMP pathway.Metabolism. 2013 Jan;62(1):79-89. doi: 10.1016/j.metabol.2012.06.011. Epub 2012 Aug 11. Metabolism. 2013. PMID: 22889511
-
Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro.Biochem J. 1997 Feb 15;322 ( Pt 1)(Pt 1):223-8. doi: 10.1042/bj3220223. Biochem J. 1997. PMID: 9078265 Free PMC article.
-
Downstream mechanisms of nitric oxide-mediated skeletal muscle glucose uptake during contraction.Am J Physiol Regul Integr Comp Physiol. 2010 Dec;299(6):R1656-65. doi: 10.1152/ajpregu.00433.2010. Epub 2010 Oct 13. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20943856
-
Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity.Mol Cell Endocrinol. 2013 Feb 25;366(2):204-14. doi: 10.1016/j.mce.2012.06.013. Epub 2012 Jul 11. Mol Cell Endocrinol. 2013. PMID: 22796442 Review.
-
Nitric oxide in modulating oxidative stress mediated skeletal muscle insulin resistance.Mol Biol Rep. 2024 Aug 29;51(1):944. doi: 10.1007/s11033-024-09874-y. Mol Biol Rep. 2024. PMID: 39210004 Review.
Cited by
-
Natriuretic peptides promote glucose uptake in a cGMP-dependent manner in human adipocytes.Sci Rep. 2018 Jan 18;8(1):1097. doi: 10.1038/s41598-018-19619-0. Sci Rep. 2018. PMID: 29348496 Free PMC article.
-
VASP increases hepatic fatty acid oxidation by activating AMPK in mice.Diabetes. 2013 Jun;62(6):1913-22. doi: 10.2337/db12-0325. Epub 2013 Jan 24. Diabetes. 2013. PMID: 23349495 Free PMC article.
-
A novel thermoregulatory role for PDE10A in mouse and human adipocytes.EMBO Mol Med. 2016 Jul 1;8(7):796-812. doi: 10.15252/emmm.201506085. Print 2016 Jul. EMBO Mol Med. 2016. PMID: 27247380 Free PMC article.
-
Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells.J Physiol. 2010 Sep 15;588(Pt 18):3551-66. doi: 10.1113/jphysiol.2010.194035. Epub 2010 Jul 19. J Physiol. 2010. PMID: 20643772 Free PMC article.
-
Effect of low-dose tadalafil once daily on glycemic control in patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, placebo-controlled pilot study.Diabetol Metab Syndr. 2022 Apr 21;14(1):56. doi: 10.1186/s13098-022-00825-w. Diabetol Metab Syndr. 2022. PMID: 35449082 Free PMC article.
References
-
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. - PubMed
-
- Roberts CK, Barnard RJ, Scheck SH, Balon TW. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol. 1997;273:E220–E225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

