Antitumoral and antimetastatic effects of metronomic chemotherapy with cyclophosphamide combined with celecoxib on murine mammary adenocarcinomas
- PMID: 20349084
- PMCID: PMC11827800
- DOI: 10.1007/s00432-010-0869-9
Antitumoral and antimetastatic effects of metronomic chemotherapy with cyclophosphamide combined with celecoxib on murine mammary adenocarcinomas
Abstract
Purpose: Metronomic chemotherapy (MCT) refers to the chronic and equally spaced administration of low doses of different chemotherapy drugs, without extended interruptions. Previously, we demonstrated the antitumor effect of MCT with cyclophosphamide (Cy) in a mouse mammary adenocarcinoma model. Herein, we investigated the therapeutic efficacy of metronomic Cy combined with celecoxib (Cel) in two murine mammary adenocarcinoma models.
Methods: Mice were s.c. challenged with M-234 p or M-406 mammary tumors and from day 5 or 8 on, respectively, treated with: (I) no treatment (controls); (II) Cy in the drinking water (25-30 mg/kg body weight/day); (III) Cel (30 mg/kg p.o.), five times/week; (IV) treated as II + III. Mice challenged i.v. with M-234 p or M-406 tumor cells received, on day 3, the same treatments.
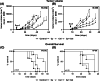
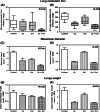
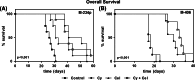
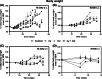
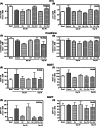
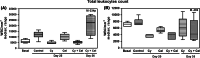
Results: We found that MCT with Cy plus Cel inhibited tumor growth decreased lung metastases, and increased the median survival time, in both tumor models, having very low toxicity. MCT with Cy combined with Cel was more effective than each monotherapy.
Conclusions: The therapeutic benefits of combined MCT with cyclophosphamide plus celecoxib on mammary adenocarcinomas together with its very low toxicity profile warrant further study in an attempt to make the translation into the clinic.
Figures

Similar articles
-
Therapeutic efficacy of metronomic chemotherapy with cyclophosphamide and doxorubicin on murine mammary adenocarcinomas.Ann Oncol. 2013 Sep;24(9):2310-6. doi: 10.1093/annonc/mdt164. Epub 2013 May 10. Ann Oncol. 2013. PMID: 23666914
-
Comparative effectiveness of two metronomic chemotherapy schedules-our experience in the preclinical field.Cancer Invest. 2014 Mar;32(3):92-8. doi: 10.3109/07357907.2013.877480. Cancer Invest. 2014. PMID: 24499110
-
Schedule dependent toxicity and efficacy of combined gemcitabine/paclitaxel treatment in mouse adenocarcinoma.J Cancer Res Clin Oncol. 2000 Aug;126(8):461-7. doi: 10.1007/PL00021282. J Cancer Res Clin Oncol. 2000. PMID: 10961389 Free PMC article.
-
Rapid COJEC versus standard induction therapies for high-risk neuroblastoma.Cochrane Database Syst Rev. 2015 May 19;2015(5):CD010774. doi: 10.1002/14651858.CD010774.pub2. Cochrane Database Syst Rev. 2015. PMID: 25989478 Free PMC article.
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
Cited by
-
Losartan improves the therapeutic effect of metronomic cyclophosphamide in triple negative mammary cancer models.Oncotarget. 2020 Aug 11;11(32):3048-3060. doi: 10.18632/oncotarget.27694. eCollection 2020 Aug 11. Oncotarget. 2020. PMID: 32850009 Free PMC article.
-
Celecoxib and octreotide synergistically ameliorate portal hypertension via inhibition of angiogenesis in cirrhotic rats.Angiogenesis. 2016 Oct;19(4):501-11. doi: 10.1007/s10456-016-9522-9. Epub 2016 Jul 5. Angiogenesis. 2016. PMID: 27380212 Free PMC article.
-
Highlights from the 1st Latin American meeting on metronomic chemotherapy and drug repositioning in oncology, 27-28 May, 2016, Rosario, Argentina.Ecancermedicalscience. 2016 Sep 6;10:672. doi: 10.3332/ecancer.2016.672. eCollection 2016. Ecancermedicalscience. 2016. PMID: 27610198 Free PMC article.
-
Potential effect of chloroquine and propranolol combination to treat colorectal and triple-negative breast cancers.Sci Rep. 2023 May 16;13(1):7923. doi: 10.1038/s41598-023-34793-6. Sci Rep. 2023. PMID: 37193722 Free PMC article.
References
-
- Andre N, Rome A, Coze C, Padovani L, Pasquier E, Camoin L, Gentet JC (2008) Metronomic etoposide/cyclophosphamide/celecoxib regimen given to children and adolescents with refractory cancer: a preliminary monocentric study. Clin Ther 30:1336–1340 - PubMed
-
- Basu GD, Pathangey LB, Tinder TL, Lagioia M, Gendler SJ, Mukherjee P (2004) Cyclooxygenase-2 inhibitor induces apoptosis in breast cancer cells in an in vivo model of spontaneous metastatic breast cancer. Mol Cancer Res 2:632–642 - PubMed
-
- Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60:1878–1886 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

