Cu-free click cycloaddition reactions in chemical biology
- PMID: 20349533
- PMCID: PMC2865253
- DOI: 10.1039/b901970g
Cu-free click cycloaddition reactions in chemical biology
Abstract
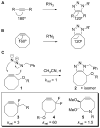
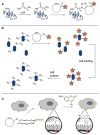
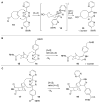
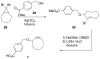
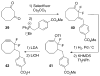
Bioorthogonal chemical reactions are paving the way for new innovations in biology. These reactions possess extreme selectivity and biocompatibility, such that their participating reagents can form covalent bonds within richly functionalized biological systems--in some cases, living organisms. This tutorial review will summarize the history of this emerging field, as well as recent progress in the development and application of bioorthogonal copper-free click cycloaddition reactions.
Figures

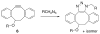
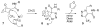



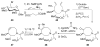

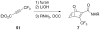
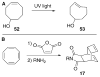
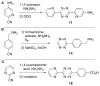
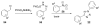
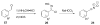
References
-
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596–2599. - PubMed
-
- Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. - PubMed
-
-
For a recent review see: Tornøe CW, Meldal M. Chem Rev. 2008;108:2952–3015.
-
-
-
For a recent review see: Sletten EM, Bertozzi CR. Angew Chem, Int Ed. 2009;48:6974–6998.
-
-
- Gaetke LM, Chow CK. Toxicology. 2003;189:147–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

