Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT
- PMID: 20350162
- PMCID: PMC2853730
- DOI: 10.1086/651662
Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT
Abstract
Background: Few data exist on respiratory virus quantitation in lower respiratory samples and detection in serum from hematopoietic cell transplant (HCT) recipients with respiratory virus-associated pneumonia.
Methods: We retrospectively identified HCT recipients with respiratory syncytial virus (RSV), parainfluenza virus, influenza virus, metapneumovirus (MPV), and coronavirus (CoV) detected in bronchoalveolar lavage (BAL) samples, and we tested stored BAL and/or serum samples by quantitative polymerase chain reaction.
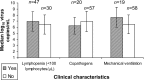
Results: In 85 BAL samples from 82 patients, median viral loads were as follows: for RSV (n = 35), 2.6 x 10(6) copies/mL; for parainfluenza virus (n = 35), 4.9 x 10(7) copies/mL; for influenza virus (n = 9), 6.8 x 10(5) copies/mL; for MPV (n = 7), 3.9 x 10(7) copies/mL; and for CoV (n = 4), 1.8 x 10(5) copies/mL. Quantitative viral load was not associated with mechanical ventilation or death. Viral RNA was detected in serum samples from 6 of 66 patients: 4 of 41 with RSV pneumonia, 1 with influenza B, and 1 with MPV/influenza A virus/CoV coinfection (influenza A virus and MPV RNA detected). RSV detection in serum was associated with high viral load in BAL samples (p = .05), and viral RNA detection in serum was significantly associated with death (adjusted rate ratio, 1.8; (p = .02).
Conclusion: Quantitative polymerase chain reaction detects high viral loads in BAL samples from HCT recipients with respiratory virus pneumonia. Viral RNA is also detectable in the serum of patients with RSV, influenza, and MPV pneumonia and may correlate with the severity of disease.
Conflict of interest statement
Potential conflicts of interest: A.P.C. has received research support from MedImmune. J.A.E. has received research support from Sanofi Pasteur, MedImmune, ADMA Biologics, Adamas Pharmaceuticals, and Novartis. M.B. has received research support from ADMA Biologics, Adamas Pharmaceuticals, and Roche Pharmaceuticals and is a consultant for Roche Pharmaceuticals and Novartis. All other authors report no potential conflicts.
Figures






References
-
- Boeckh M, Berrey MM, Bowden RA, Crawford SW, Balsley J, Corey L. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184:350–354. - PubMed
-
- Champlin RE, Whimbey E. Community respiratory virus infections in bone marrow transplant recipients: the M.D. Anderson Cancer Center experience. Biol Blood Marrow Transplant. 2001;7(suppl):8S–10S. - PubMed
-
- Ljungman P, Gleaves CA, Meyers JD. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant. 1989;4:35–40. - PubMed
-
- Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–796. - PMC - PubMed
-
- Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–578. - PubMed

