Effect of vaccination with modified vaccinia Ankara (ACAM3000) on subsequent challenge with Dryvax
- PMID: 20350190
- PMCID: PMC3023456
- DOI: 10.1086/651560
Effect of vaccination with modified vaccinia Ankara (ACAM3000) on subsequent challenge with Dryvax
Abstract
Background: Despite the success of smallpox vaccination, the immunological correlates of protection are not fully understood. To investigate this question, we examined the effect of immunization with modified vaccinia Ankara (MVA) on subsequent challenge with replication-competent vaccinia virus (Dryvax).
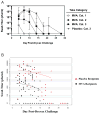
Methods: Dryvax challenge by scarification was conducted in 36 healthy subjects who had received MVA (n = 29) or placebo (n = 7) in a previous study of doses and routes of immunization. Subjects were followed up for clinical take, viral shedding, and immune responses.
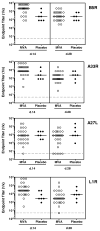
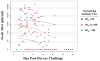
Results: MVA administration attenuated clinical takes in 21 (72%) of 29 subjects, compared with 0 of 7 placebo recipients (P = .001). Attenuation was most significant in MVA groups that received 1 x 10(7) median tissue culture infective doses (TCID(50)) intradermally (P = .001) and 1 x 10(7) TCID(50) intramuscularly (P = .001). Both duration and peak titer of viral shedding were reduced in MVA recipients. Peak neutralizing antibody responses to vaccinia virus or MVA previously induced by MVA immunization were associated with attenuated takes (P = .02) and reduced duration (P = .001) and titer (P = .005) of viral shedding.
Conclusions: MVA immunization results in clinical and virologic protection against Dryvax challenge. Protection is associated with prior induction of neutralizing antibodies to MVA or vaccinia virus. MVA administered intradermally has protective and immunologic responses similar to those of a 10-fold-higher dose given subcutaneously.
Trial registration: ClinicalTrials.gov NCT00133575.
Conflict of interest statement
Figures



References
-
- Fenner F, Henderson D, Arita I, Jezek Z. Smallpox and Its Eradication. World Health Organization; 1988. p. 307.
-
- McCurdy LH, Larkin BD, Martin JE, Graham BS. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin Infect Dis. 2004;38:1749–53. - PubMed
-
- Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289:3283–9. - PubMed
-
- Eckart RE, Love SS, Atwood JE, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–5. - PubMed

