Distinct genotypic profiles of the two major clades of Mycobacterium africanum
- PMID: 20350321
- PMCID: PMC2859774
- DOI: 10.1186/1471-2334-10-80
Distinct genotypic profiles of the two major clades of Mycobacterium africanum
Abstract
Background: Mycobacterium tuberculosis is the principal etiologic agent of human tuberculosis (TB) and a member of the M. tuberculosis complex (MTC). Additional MTC species that cause TB in humans and other mammals include Mycobacterium africanum and Mycobacterium bovis. One result of studies interrogating recently identified MTC phylogenetic markers has been the recognition of at least two distinct lineages of M. africanum, known as West African-1 and West African-2.
Methods: We screened a blinded non-random set of MTC strains isolated from TB patients in Ghana (n = 47) for known chromosomal region-of-difference (RD) loci and single nucleotide polymorphisms (SNPs). A MTC PCR-typing panel, single-target standard PCR, multi-primer PCR, PCR-restriction fragment analysis, and sequence analysis of amplified products were among the methods utilized for the comparative evaluation of targets and identification systems. The MTC distributions of novel SNPs were characterized in the both the Ghana collection and two other diverse collections of MTC strains (n = 175 in total).
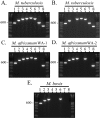
Results: The utility of various polymorphisms as species-, lineage-, and sublineage-defining phylogenetic markers for M. africanum was determined. Novel SNPs were also identified and found to be specific to either M. africanum West African-1 (Rv1332(523); n = 32) or M. africanum West African-2 (nat(751); n = 27). In the final analysis, a strain identification approach that combined multi-primer PCR targeting of the RD loci RD9, RD10, and RD702 was the most simple, straight-forward, and definitive means of distinguishing the two clades of M. africanum from one another and from other MTC species.
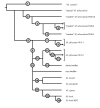
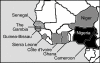
Conclusion: With this study, we have organized a series of consistent phylogenetically-relevant markers for each of the distinct MTC lineages that share the M. africanum designation. A differential distribution of each M. africanum clade in Western Africa is described.
Figures
References
-
- Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci USA. 2002;99(6):3684–3689. doi: 10.1073/pnas.052548299. - DOI - PMC - PubMed
-
- Cousins D, Bastida R, Cataldi A, Quse V, Redrobe S, Dow S, Duignan P, Murray A, Dupont C, Ahmed N, Collins D, Butler W, Dawson D, Rodríguez D, Loureiro J, Romano M, Alito A, Zumarraga M, Bernardelli A. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int J Syst Evol Microbiol. 2003;53(5):1305–1314. doi: 10.1099/ijs.0.02401-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

