A randomized, double-blind, placebo-controlled study of high-dose bosentan in patients with stage IV metastatic melanoma receiving first-line dacarbazine chemotherapy
- PMID: 20350333
- PMCID: PMC2856553
- DOI: 10.1186/1476-4598-9-69
A randomized, double-blind, placebo-controlled study of high-dose bosentan in patients with stage IV metastatic melanoma receiving first-line dacarbazine chemotherapy
Abstract
Background: The endothelin system is implicated in the pathogenesis of melanoma. We evaluated the effects of bosentan - a dual endothelin receptor antagonist - in patients receiving first-line dacarbazine therapy for stage IV metastatic cutaneous melanoma in a phase 2, proof-of-concept study.
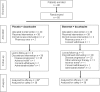
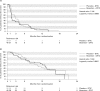
Results: Eligible patients had metastatic cutaneous melanoma naïve to chemotherapy or immunotherapy, no central nervous system involvement, and serum lactate dehydrogenase <1.5 x upper limit of normal. Treatment comprised bosentan 500 mg twice daily or matching placebo, in addition to dacarbazine 1000 mg/m2 every three weeks. Eighty patients were randomized (double-blind) and 38 in each group received study treatment. Median time to tumor progression (primary endpoint) was not significantly different between the two groups (placebo, 2.8 months; bosentan, 1.6 months; bosentan/placebo hazard ratio, 1.144; 95% CI, 0.717-1.827; p = 0.5683). Incidences of most adverse events and clinically relevant increases in hepatic transaminases were similar between treatment groups although hemoglobin decrease to >8 and < or = 10 g/dL and < or = 8 g/dL was more common in the bosentan group.
Conclusions: In patients receiving dacarbazine as first-line chemotherapy for metastatic melanoma, the addition of high-dose bosentan had no effect on time to tumor progression or other efficacy parameters. There were no unexpected safety findings.
Trial registration: This study is registered in ClinicalTrials.gov under the unique identifier NCT01009177.
Figures
References
-
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. - PubMed
-
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

