Spatial working memory deficits in GluA1 AMPA receptor subunit knockout mice reflect impaired short-term habituation: evidence for Wagner's dual-process memory model
- PMID: 20350557
- PMCID: PMC2938569
- DOI: 10.1016/j.neuropsychologia.2010.03.018
Spatial working memory deficits in GluA1 AMPA receptor subunit knockout mice reflect impaired short-term habituation: evidence for Wagner's dual-process memory model
Abstract
Genetically modified mice, lacking the GluA1 AMPA receptor subunit, are impaired on spatial working memory tasks, but display normal acquisition of spatial reference memory tasks. One explanation for this dissociation is that working memory, win-shift performance engages a GluA1-dependent, non-associative, short-term memory process through which animals choose relatively novel arms in preference to relatively familiar options. In contrast, spatial reference memory, as exemplified by the Morris water maze task, reflects a GluA1-independent, associative, long-term memory mechanism. These results can be accommodated by Wagner's dual-process model of memory in which short and long-term memory mechanisms exist in parallel and, under certain circumstances, compete with each other. According to our analysis, GluA1(-/-) mice lack short-term memory for recently experienced spatial stimuli. One consequence of this impairment is that these stimuli should remain surprising and thus be better able to form long-term associative representations. Consistent with this hypothesis, we have recently shown that long-term spatial memory for recently visited locations is enhanced in GluA1(-/-) mice, despite impairments in hippocampal synaptic plasticity. Taken together, these results support a role for GluA1-containing AMPA receptors in short-term habituation, and in modulating the intensity or perceived salience of stimuli.
Figures
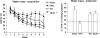
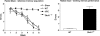
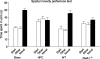


Similar articles
-
The role of habituation in hippocampus-dependent spatial working memory tasks: evidence from GluA1 AMPA receptor subunit knockout mice.Hippocampus. 2012 May;22(5):981-94. doi: 10.1002/hipo.20896. Epub 2010 Dec 1. Hippocampus. 2012. PMID: 21125585 Free PMC article. Review.
-
Enhanced long-term and impaired short-term spatial memory in GluA1 AMPA receptor subunit knockout mice: evidence for a dual-process memory model.Learn Mem. 2009 May 23;16(6):379-86. doi: 10.1101/lm.1339109. Print 2009 Jun. Learn Mem. 2009. PMID: 19470654 Free PMC article.
-
Dissociations within short-term memory in GluA1 AMPA receptor subunit knockout mice.Behav Brain Res. 2011 Oct 10;224(1):8-14. doi: 10.1016/j.bbr.2011.05.016. Epub 2011 May 27. Behav Brain Res. 2011. PMID: 21641937 Free PMC article.
-
Deletion of the GluA1 AMPA receptor subunit alters the expression of short-term memory.Learn Mem. 2011 Feb 16;18(3):128-31. doi: 10.1101/lm.2014911. Print 2011 Mar. Learn Mem. 2011. PMID: 21325433 Free PMC article.
-
The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory.Prog Brain Res. 2008;169:159-78. doi: 10.1016/S0079-6123(07)00009-X. Prog Brain Res. 2008. PMID: 18394473 Review.
Cited by
-
Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis.Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15625-30. doi: 10.1073/pnas.1005807107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713712 Free PMC article.
-
Therapeutic treatment with the anti-inflammatory drug candidate MW151 may partially reduce memory impairment and normalizes hippocampal metabolic markers in a mouse model of comorbid amyloid and vascular pathology.PLoS One. 2022 Jan 26;17(1):e0262474. doi: 10.1371/journal.pone.0262474. eCollection 2022. PLoS One. 2022. PMID: 35081152 Free PMC article.
-
AMPA Receptors in Synaptic Plasticity, Memory Function, and Brain Diseases.Cell Mol Neurobiol. 2025 Jan 22;45(1):14. doi: 10.1007/s10571-024-01529-7. Cell Mol Neurobiol. 2025. PMID: 39841263 Free PMC article. Review.
-
Working memory and the homeostatic control of brain adenosine by adenosine kinase.Neuroscience. 2012 Jun 28;213:81-92. doi: 10.1016/j.neuroscience.2012.03.051. Epub 2012 Apr 19. Neuroscience. 2012. PMID: 22521820 Free PMC article.
-
The effects of preventative cannabidiol in a male neuregulin 1 mouse model of schizophrenia.Front Cell Neurosci. 2022 Nov 3;16:1010478. doi: 10.3389/fncel.2022.1010478. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36406747 Free PMC article.
References
-
- Aggleton J.P., Neave N., Nagle S., Sahgal A. A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: Evidence of a double dissociation between frontal and cingulum bundle contributions. Journal of Neuroscience. 1995;15(11):7270–7281. - PMC - PubMed
-
- Baddeley A.D., Hitch G. Working memory. In: Bower G.H., editor. Vol. 8. Academic Press; New York: 1974. pp. 47–89. (The psychology of learning and motivation: Advances in research and theory).
-
- Bannerman D.M., Deacon R.M., Brady S., Bruce A., Sprengel R., Seeburg P.H. A comparison of GluR-A-deficient and wild-type mice on a test battery assessing sensorimotor, affective, and cognitive behaviors. Behavioral Neuroscience. 2004;118(3):643–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

