Frequency-dependent neural activity, CBF, and BOLD fMRI to somatosensory stimuli in isoflurane-anesthetized rats
- PMID: 20350603
- PMCID: PMC2883664
- DOI: 10.1016/j.neuroimage.2010.03.064
Frequency-dependent neural activity, CBF, and BOLD fMRI to somatosensory stimuli in isoflurane-anesthetized rats
Abstract
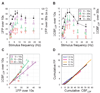
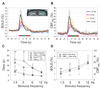
Inhalation anesthetics (e.g. isoflurane) are preferable for longitudinal fMRI experiments in the same animals. We previously implemented isoflurane anesthesia for rodent forepaw stimulation studies, and optimized the stimulus parameters with short stimuli (1-3-s long stimulation with ten electric pulses). These parameters, however, may not be applicable for long periods of stimulation because repetitive stimuli induce neural adaptation. Here we evaluated frequency-dependent responses (pulse width of 1.0 ms and current of 1.5 mA) for 30-s long stimulation under 1.3-1.5% isoflurane anesthesia. The cerebral blood flow (CBF) response (using laser Doppler flowmetry: CBF(LDF)) and field potential (FP) changes were simultaneously measured for nine stimulus frequencies (1-24 Hz). CBF (using arterial spin labeling: CBF(ASL)) and blood oxygenation level dependent (BOLD) fMRI responses were measured at 9.4 T for four stimulus frequencies (1.5-12 Hz). Higher stimulus frequencies (12-24 Hz) produced a larger FP per unit time initially, but decreased more rapidly later due to neural adaptation effects. On the other hand, lower stimulus frequencies (1-3 Hz) induced smaller, but sustained FP activities over the entire stimulus period. Similar frequency-dependencies were observed in CBF(LDF), CBF(ASL) and BOLD responses. A linear relationship between FP and CBF(LDF) was observed for all stimulus frequencies. Stimulation frequency for the maximal cumulative neural and hemodynamic changes is dependent on stimulus duration; 8-12 Hz for short stimulus durations (<10s) and 6-8 Hz for 30-s stimulation. Our findings suggest that neural adaptation should be considered in determining the somatosensory stimulation frequency and duration under isoflurane anesthesia.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Banoub M, Tetzlaff JE, Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials: implications for perioperative monitoring. Anesthesiology. 2003;99:716–737. - PubMed
-
- Barbier EL, Silva AC, Kim HJ, Williams DS, Koretsky AP. Perfusion analysis using dynamic arterial spin labeling (DASL) Magn Reson Med. 1999;41:299–308. - PubMed
-
- Bock C, Krep H, Brinker G, Hoehn-Berlage M. Brainmapping of alpha-chloralose anesthetized rats with T2*-weighted imaging: distinction between the representation of the forepaw and hindpaw in the somatosensory cortex. NMR Biomed. 1998;11:115–119. - PubMed
-
- Brinker G, Bock C, Busch E, Krep H, Hossmann KA, Hoehn-Berlage M. Simultaneous recording of evoked potentials and T2*-weighted MR images during somatosensory stimulation of rat. Magn Reson Med. 1999;41:469–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

