Direct voltage control of endogenous lysophosphatidic acid G-protein-coupled receptors in Xenopus oocytes
- PMID: 20351041
- PMCID: PMC2887987
- DOI: 10.1113/jphysiol.2009.183418
Direct voltage control of endogenous lysophosphatidic acid G-protein-coupled receptors in Xenopus oocytes
Abstract
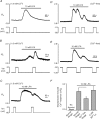
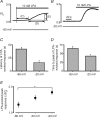
Lysophosphatidic acid (LPA) G-protein-coupled receptors (GPCRs) play important roles in a variety of physiological and pathophysiological processes, including cell proliferation, angiogenesis, central nervous system development and carcinogenesis. Whilst many ion channels and transporters are recognized to be controlled by a change in cell membrane potential, little is known about the voltage dependence of other proteins involved in cell signalling. Here, we show that the InsP(3)-mediated Ca(2+) response stimulated by the endogenous LPA GPCR in Xenopus oocytes is potentiated by membrane depolarization. Depolarization was able to repetitively stimulate transient [Ca(2+)](i) increases after the initial agonist-evoked response. In addition, the initial rate and amplitude of the LPA-dependent Ca(2+) response were significantly modulated by the steady holding potential over the physiological range, such that the response to LPA was potentiated at depolarized potentials and inhibited at hyperpolarized potentials. Enhancement of LPA receptor-evoked Ca(2+) mobilization by membrane depolarization was observed over a wide range of agonist concentrations. Importantly, the amplitude of the depolarization-evoked intracellular Ca(2+) increase displayed an inverse relationship with agonist concentration such that the greatest effect of voltage was observed at near-threshold levels of agonist. Voltage-dependent Ca(2+) release was not induced by direct elevation of InsP(3) or by activation of heterotrimeric G-proteins in the absence of agonist, indicating that the LPA GPCR itself represents the primary site of action of membrane voltage. This novel modulation of LPA signalling by membrane potential may have important consequences for control of Ca(2+) signals both in excitable and non-excitable tissues.
Figures

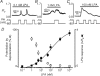
 , where x0 is the middle value, p is the power and A1 and A2 are the initial and final Y values, respectively. Each point is the mean and
, where x0 is the middle value, p is the power and A1 and A2 are the initial and final Y values, respectively. Each point is the mean and 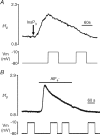
References
-
- An S, Bleu T, Zheng Y, Goetzl EJ. Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization. Mol Pharmacol. 1998;54:881–888. - PubMed
-
- Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem. 2001;292:287–295. - PubMed
-
- Ben Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. - PubMed
-
- Ben Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278:22482–22491. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

