Separate gating mechanisms mediate the regulation of K2P potassium channel TASK-2 by intra- and extracellular pH
- PMID: 20351106
- PMCID: PMC2878019
- DOI: 10.1074/jbc.M110.107060
Separate gating mechanisms mediate the regulation of K2P potassium channel TASK-2 by intra- and extracellular pH
Abstract
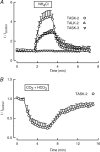
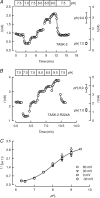
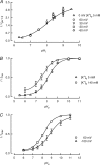
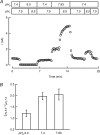
TASK-2 (KCNK5 or K(2P)5.1) is a background K(+) channel that is opened by extracellular alkalinization and plays a role in renal bicarbonate reabsorption and central chemoreception. Here, we demonstrate that in addition to its regulation by extracellular protons (pH(o)) TASK-2 is gated open by intracellular alkalinization. The following pieces of evidence suggest that the gating process controlled by intracellular pH (pH(i)) is independent from that under the command of pH(o). It was not possible to overcome closure by extracellular acidification by means of intracellular alkalinization. The mutant TASK-2-R224A that lacks sensitivity to pH(o) had normal pH(i)-dependent gating. Increasing extracellular K(+) concentration acid shifts pH(o) activity curve of TASK-2 yet did not affect pH(i) gating of TASK-2. pH(o) modulation of TASK-2 is voltage-dependent, whereas pH(i) gating was not altered by membrane potential. These results suggest that pH(o), which controls a selectivity filter external gate, and pH(i) act at different gating processes to open and close TASK-2 channels. We speculate that pH(i) regulates an inner gate. We demonstrate that neutralization of a lysine residue (Lys(245)) located at the C-terminal end of transmembrane domain 4 by mutation to alanine abolishes gating by pH(i). We postulate that this lysine acts as an intracellular pH sensor as its mutation to histidine acid-shifts the pH(i)-dependence curve of TASK-2 as expected from its lower pK(a). We conclude that intracellular pH, together with pH(o), is a critical determinant of TASK-2 activity and therefore of its physiological function.
Figures



References
-
- Goldstein S. A., Bayliss D. A., Kim D., Lesage F., Plant L. D., Rajan S. (2005) Pharmacol. Rev. 57, 527–540 - PubMed
-
- Reyes R., Duprat F., Lesage F., Fink M., Salinas M., Farman N., Lazdunski M. (1998) J. Biol. Chem. 273, 30863–30869 - PubMed
-
- Kim Y., Bang H., Kim D. (2000) J. Biol. Chem. 275, 9340–9347 - PubMed
-
- Lopes C. M., Zilberberg N., Goldstein S. A. (2001) J. Biol. Chem. 276, 24449–24452 - PubMed
-
- Rajan S., Wischmeyer E., Xin, Liu G., Preisig-Müller R., Daut J., Karschin A., Derst C. (2000) J. Biol. Chem. 275, 16650–16657 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

