Mechanism of intraparticle synthesis of the rotavirus double-stranded RNA genome
- PMID: 20351108
- PMCID: PMC2881735
- DOI: 10.1074/jbc.R110.117671
Mechanism of intraparticle synthesis of the rotavirus double-stranded RNA genome
Abstract
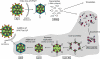
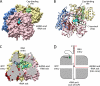
Rotaviruses perform the remarkable tasks of transcribing and replicating 11 distinct double-stranded RNA genome segments within the confines of a subviral particle. Multiple viral polymerases are tethered to the interior of a particle, each dedicated to a solitary genome segment but acting in synchrony to synthesize RNA. Although the rotavirus polymerase specifically recognizes RNA templates in the absence of other proteins, its enzymatic activity is contingent upon interaction with the viral capsid. This intraparticle strategy of RNA synthesis helps orchestrate the concerted packaging and replication of the viral genome. Here, we review our current understanding of rotavirus RNA synthetic mechanisms.
Figures

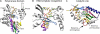
Similar articles
-
Assortment and packaging of the segmented rotavirus genome.Trends Microbiol. 2011 Mar;19(3):136-44. doi: 10.1016/j.tim.2010.12.002. Epub 2010 Dec 31. Trends Microbiol. 2011. PMID: 21195621 Free PMC article. Review.
-
In Vitro Double-Stranded RNA Synthesis by Rotavirus Polymerase Mutants with Lesions at Core Shell Contact Sites.J Virol. 2019 Sep 30;93(20):e01049-19. doi: 10.1128/JVI.01049-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31341048 Free PMC article.
-
Rotavirus RNA polymerase requires the core shell protein to synthesize the double-stranded RNA genome.J Virol. 1997 Dec;71(12):9618-26. doi: 10.1128/JVI.71.12.9618-9626.1997. J Virol. 1997. PMID: 9371626 Free PMC article.
-
Rotavirus replication: plus-sense templates for double-stranded RNA synthesis are made in viroplasms.J Virol. 2004 Jul;78(14):7763-74. doi: 10.1128/JVI.78.14.7763-7774.2004. J Virol. 2004. PMID: 15220450 Free PMC article.
-
Rotavirus NSP2: A Master Orchestrator of Early Viral Particle Assembly.Viruses. 2024 May 21;16(6):814. doi: 10.3390/v16060814. Viruses. 2024. PMID: 38932107 Free PMC article. Review.
Cited by
-
Mycoreovirus genome alterations: similarities to and differences from rearrangements reported for other reoviruses.Front Microbiol. 2012 Jun 1;3:186. doi: 10.3389/fmicb.2012.00186. eCollection 2012. Front Microbiol. 2012. PMID: 22675320 Free PMC article.
-
Interaction between a Unique Minor Protein and a Major Capsid Protein of Bluetongue Virus Controls Virus Infectivity.J Virol. 2018 Jan 17;92(3):e01784-17. doi: 10.1128/JVI.01784-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29142128 Free PMC article.
-
Structure of the T=13 capsid of infectious pancreatic necrosis virus (IPNV)-a salmonid birnavirus.J Virol. 2025 Feb 25;99(2):e0145424. doi: 10.1128/jvi.01454-24. Epub 2025 Jan 16. J Virol. 2025. PMID: 39817769 Free PMC article.
-
The battle between rotavirus and its host for control of the interferon signaling pathway.PLoS Pathog. 2013 Jan;9(1):e1003064. doi: 10.1371/journal.ppat.1003064. Epub 2013 Jan 24. PLoS Pathog. 2013. PMID: 23359266 Free PMC article. Review.
-
High-resolution comparative atomic structures of two Giardiavirus prototypes infecting G. duodenalis parasite.PLoS Pathog. 2024 Apr 10;20(4):e1012140. doi: 10.1371/journal.ppat.1012140. eCollection 2024 Apr. PLoS Pathog. 2024. PMID: 38598600 Free PMC article.
References
-
- Mertens P. P., Diprose J. (2004) Virus Res. 101, 29–43 - PubMed
-
- Patton J. T., Vasquez-Del Carpio R., Tortorici M. A., Taraporewala Z. F. (2007) Adv. Virus Res. 69, 167–201 - PubMed
-
- McDonald S. M., Patton J. T. (2009) in Viral Genome Replication ( Cameron C. E., Gotte M., Raney K. D. eds) pp.201– 224, Springer Science+Business Media, New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

